Rapid, Validated UPLC-MS/MS Method for Determination of Glibenclamide in Rat Plasma
- PMID: 30245720
- PMCID: PMC6139228
- DOI: 10.1155/2018/2569027
Rapid, Validated UPLC-MS/MS Method for Determination of Glibenclamide in Rat Plasma
Erratum in
-
Erratum to "Rapid, Validated UPLC-MS/MS Method for Determination of Glibenclamide in Rat Plasma".Int J Anal Chem. 2019 Feb 3;2019:6470528. doi: 10.1155/2019/6470528. eCollection 2019. Int J Anal Chem. 2019. PMID: 30853988 Free PMC article.
Abstract
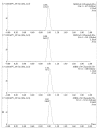
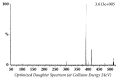
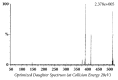
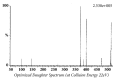
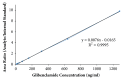
Quick and specific bioanalytical methods are required for analyzing drugs in biological samples. A simple, quick, sensitive, and specific UPLC-MS/MS method was developed and validated for glibenclamide determination in plasma samples. The plasma samples were processed by protein precipitation technique. Glimepiride was used as internal standard (IS). Glibenclamide and glimepiride were eluted on C18 column (Acquity UPLC®BEH). Mobile phase consisting of acetonitrile (0.1% formic acid) and water (0.1% formic acid) was pumped in binary gradient mode at flow rate of 150 μL/min. Glibenclamide and IS elution time was about 1.0 min, and total run time was 2.0 min. The mass spectrometer (triple-quadrupole) was operated in positive electrospray ionization mode. Sodium adducts [M + Na]+ of glibenclamide and IS were monitored in MRM mode. A linear calibration curve was obtained in the range of 10-1280 ng/mL, with regression equation Y = 0.0076 X - 0.0165 and linear regression coefficient r2 = 0.999. Lower limit of quantitation was 10 ng/mL. Accuracy of the method at LQC, MQC, and HQC was 109.7% (± 6.7), 93.6% (± 0.4), and 99.3% (± 1.9), respectively. The coefficient of variation for precision at all QC concentrations was less than 6%. Recovery at LLQC, MQC, and HQC was 104.2% (± 4.9), 100.6% (± 0.9), and 102.9% (± 5.8), respectively. The method was successfully implemented for pharmacokinetic investigations (in-house data).
Figures

References
-
- Khatri J., Qassim S., Abed O., Abraham B., Al-Lami A., Masood S. A novel extractionless HPLC fluorescence method for the determination of glyburide in the human plasma: Application to a bioequivalence study. Journal of Pharmacy & Pharmaceutical Sciences. 2001;4(2):201–206. - PubMed
-
- Hoizey G., Lamiable D., Trenque T., et al. Identification and quantification of 8 sulfonylureas with clinical toxicology interest by liquid chromatography-ion-trap tandem mass spectrometry and library searching. Clinical Chemistry. 2005;51(9):1666–1672. doi: 10.1373/clinchem.2005.050864. - DOI - PubMed
-
- Venkatesh P., Harisudhan T., Choudhury H., Mullangi R., Srinivas N. R. Simultaneous estimation of six anti-diabetic drugs - Glibenclamide, gliclazide, glipizide, pioglitazone, repaglinide and rosiglitazone: Development of a novel HPLC method for use in the analysis of pharmaceutical formulations and its application to human plasma assay. Biomedical Chromatography. 2006;20(10):1043–1048. doi: 10.1002/bmc.635. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

