Targeted α-therapy of prostate cancer using radiolabeled PSMA inhibitors: a game changer in nuclear medicine
- PMID: 30245917
- PMCID: PMC6146164
Targeted α-therapy of prostate cancer using radiolabeled PSMA inhibitors: a game changer in nuclear medicine
Abstract
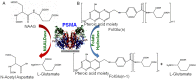
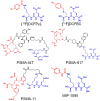
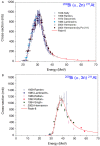
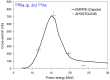
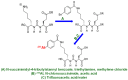
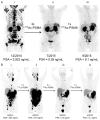
Prostate cancer (PCa) is one of the most common malignancies in men and is a major contributor to cancer related deaths worldwide. Metastatic spread and disease progression under androgen deprivation therapy signify the onset of metastatic castration resistant prostate cancer (mCRPCa)-the lethal form of the disease, which severely deteriorates the quality of life of patients. Over the last decade, tremendous progress has been made toward identifying appropriate molecular targets that could enable efficient in vivo targeting for non-invasive imaging and therapy of mCPRCa. In this context, a promising enzymatic target is prostate specific membrane antigen (PSMA), which is overexpressed on PCa cells, in proportion to the stage and grade of the tumor progression. This is especially relevant for mCRPCa, which has significant overexpression of PSMA. For therapy of mCRPCa, several nuclear medicine clinics all over the world have confirmed that 177Lu-labeled-PSMA enzyme inhibitors (177Lu-PSMA-617 and 177Lu-PSMA I&T) have a favorable dosimetry and convincing therapeutic response. However, ~30% of patients were found to be short or non-responders and dose escalation was severely limited by chronic hematological toxicity. Such limitations could be better overcome by targeted alpha therapy (TAT) which has the potential to bring a paradigm shift in treatment of mCRPCa patients. This concise review presents an overview of the successes and challenges currently faced in TAT of mCRPCa using radiolabeled PSMA inhibitors. The preclinical and clinical data reported to date are quite promising, and it is expected that this therapeutic modality will play a pivotal role in advanced stage PCa management in the foreseeable future.
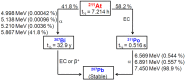
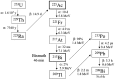
Keywords: 211At; 213Bi; 225Ac; PSMA; metastasis; prostate cancer; targeted alpha therapy.
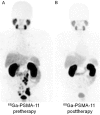
Figures


References
-
- Domachevsky L, Goldberg N, Bernstine H, Nidam M, Groshar D. Quantitative characterisation of clinically significant intra-prostatic cancer by prostate-specific membrane antigen (PSMA) expression and cell density on PSMA-11. Eur Radiol. 2018 [Epub ahead of print] - PubMed
-
- Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, Brawley OW, Wender RC. Cancer screening in the United States, 2018: a review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297–316. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
