Therapies and outcomes of congenital hyperinsulinism-induced hypoglycaemia
- PMID: 30246418
- PMCID: PMC6585719
- DOI: 10.1111/dme.13823
Therapies and outcomes of congenital hyperinsulinism-induced hypoglycaemia
Abstract
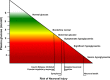
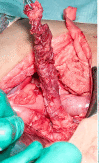
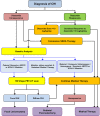
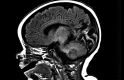
Congenital hyperinsulinism is a rare disease, but is the most frequent cause of persistent and severe hypoglycaemia in early childhood. Hypoglycaemia caused by excessive and dysregulated insulin secretion (hyperinsulinism) from disordered pancreatic β cells can often lead to irreversible brain damage with lifelong neurodisability. Although congenital hyperinsulinism has a genetic cause in a significant proportion (40%) of children, often being the result of mutations in the genes encoding the KATP channel (ABCC8 and KCNJ11), not all children have severe and persistent forms of the disease. In approximately half of those without a genetic mutation, hyperinsulinism may resolve, although timescales are unpredictable. From a histopathology perspective, congenital hyperinsulinism is broadly grouped into diffuse and focal forms, with surgical lesionectomy being the preferred choice of treatment in the latter. In contrast, in diffuse congenital hyperinsulinism, medical treatment is the best option if conservative management is safe and effective. In such cases, children receiving treatment with drugs, such as diazoxide and octreotide, should be monitored for side effects and for signs of reduction in disease severity. If hypoglycaemia is not safely managed by medical therapy, subtotal pancreatectomy may be required; however, persistent hypoglycaemia may continue after surgery and diabetes is an inevitable consequence in later life. It is important to recognize the negative cognitive impact of early-life hypoglycaemia which affects half of all children with congenital hyperinsulinism. Treatment options should be individualized to the child/young person with congenital hyperinsulinism, with full discussion regarding efficacy, side effects, outcomes and later life impact.
© 2018 The Authors. Diabetic Medicine published by John Wiley & Sons Ltd on behalf of Diabetes UK.
Figures

References
-
- Banerjee I, Avatapalle B, Padidela R, Stevens A, Cosgrove K, Clayton P, Dunne M. Integrating genetic and imaging investigations into the clinical management of congenital hyperinsulinism. Clin Endocrinol (Oxf) 2013; 78: 803–813. - PubMed
-
- Shah P, Rahman SA, Demirbilek H, Güemes M, Hussain K. Hyperinsulinaemic hypoglycaemia in children and adults. Lancet Diabetes Endocrinol. 2017; 5: 729–742. - PubMed
-
- Dunne MJ, Cosgrove KE, Shepherd RM, Aynsley‐Green A, Lindley KJ. Hyperinsulinism in infancy: from basic science to clinical disease. Physiol Rev 2004; 84: 239–275. - PubMed
-
- Menni F, de Lonlay P, Sevin C, Touati G, Peigne C, Barbier V et al Neurologic outcomes of 90 neonates and infants with persistent hyperinsulinemic hypoglycaemia. Pediatrics 2001; 107: 476–479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

