Breast cancer metastasis to gynaecological organs: a clinico-pathological and molecular profiling study
- PMID: 30246500
- PMCID: PMC6317061
- DOI: 10.1002/cjp2.118
Breast cancer metastasis to gynaecological organs: a clinico-pathological and molecular profiling study
Abstract
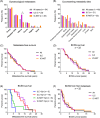
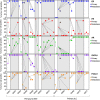
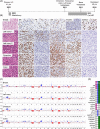
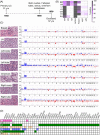
Breast cancer metastasis to gynaecological organs is an understudied pattern of tumour spread. We explored clinico-pathological and molecular features of these metastases to better understand whether this pattern of dissemination is organotropic or a consequence of wider metastatic dissemination. Primary and metastatic tumours from 54 breast cancer patients with gynaecological metastases were analysed using immunohistochemistry, DNA copy-number profiling, and targeted sequencing of 386 cancer-related genes. The median age of primary tumour diagnosis amongst patients with gynaecological metastases was significantly younger compared to a general breast cancer population (46.5 versus 60 years; p < 0.0001). Median age at metastatic diagnosis was 54.4, time to progression was 4.8 years (range 0-20 years), and survival following a diagnosis of metastasis was 1.95 years (range 0-18 years). Patients had an average of five involved sites (most frequently ovary, fallopian tube, omentum/peritoneum), with fewer instances of spread to the lungs, liver, or brain. Invasive lobular histology and luminal A-like phenotype were over-represented in this group (42.8 and 87.5%, respectively) and most patients had involved axillary lymph nodes (p < 0.001). Primary tumours frequently co-expressed oestrogen receptor cofactors (GATA3, FOXA1) and harboured amplifications at 8p12, 8q24, and 11q13. In terms of phenotype conversion, oestrogen receptor status was generally maintained in metastases, FOXA1 increased, and expression of progesterone receptor, androgen receptor, and GATA3 decreased. ESR1 and novel AR mutations were identified. Metastasis to gynaecological organs is a complication frequently affecting young women with invasive lobular carcinoma and luminal A-like breast cancer, and hence may be driven by sustained hormonal signalling. Molecular analyses reveal a spectrum of factors that could contribute to de novo or acquired resistance to therapy and disease progression.
Keywords: breast cancer; genomics; immunophenotyping; luminal subtype; metastasis; ovary.
© 2018 The Authors. The Journal of Pathology: Clinical Research published by The Pathological Society of Great Britain and Ireland and John Wiley & Sons Ltd.
Figures
References
-
- Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol 1983; 23: 175–180. - PubMed
-
- Hess KR, Varadhachary GR, Taylor SH, et al Metastatic patterns in adenocarcinoma. Cancer 2006; 106: 1624–1633. - PubMed
-
- Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery 1993; 114: 637–641 discussion 41‐2. - PubMed
-
- Lamovec J, Bracko M. Metastatic pattern of infiltrating lobular carcinoma of the breast: an autopsy study. J Surg Oncol 1991; 48: 28–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

