The Cephalopod Large Brain Enigma: Are Conserved Mechanisms of Stem Cell Expansion the Key?
- PMID: 30246785
- PMCID: PMC6110919
- DOI: 10.3389/fphys.2018.01160
The Cephalopod Large Brain Enigma: Are Conserved Mechanisms of Stem Cell Expansion the Key?
Abstract
Within the clade of mollusks, cephalopods have developed an unusually large and complex nervous system. The increased complexity of the cephalopod centralized "brain" parallels an amazing amount of complex behaviors that culminate in one order, the octopods. The mechanisms that enable evolution of expanded brains in invertebrates remain enigmatic. While expression mapping of known molecular pathways demonstrated the conservation of major neurogenesis pathways and revealed neurogenic territories, it did not explain why cephalopods could massively increase their brain size compared to other mollusks. Such an increase is reminiscent of the expansion of the cerebral cortex in mammalians, which have enlarged their number and variety of neurogenic stem cells. We hypothesize that similar mechanisms might be at play in cephalopods and that focusing on the stem cell biology of cephalopod neurogenesis and genetic innovations might be smarter strategies to uncover the mechanism that has driven cephalopod brain expansion.
Keywords: brain development; cephalopod; invertebrate neuron; neurogenesis; stem cell.
Figures
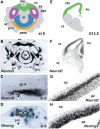
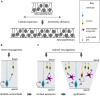
References
-
- Altman J. (2011). “The discovery of adult mammalian neurogenesis,” in Neurogenesis in the Adult Brain I, eds Seki T., Sawamoto K., Parent J. M., Alvarez-Buylla A. (Dordrecht: Springer; ), 10.1007/978-4-431-53933-9_1 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

