Differential response of pineal microglia to surgical versus pharmacological stimuli
- PMID: 30246867
- PMCID: PMC6196128
- DOI: 10.1002/cne.24505
Differential response of pineal microglia to surgical versus pharmacological stimuli
Abstract
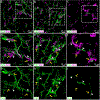
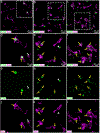
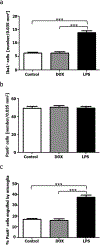
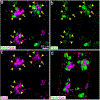
Microglial cells are one of the interstitial elements of the pineal gland (PG). We recently reported the pattern of microglia colonization and activation, and microglia-Pax6+ cell interactions during normal pineal ontogeny. Here, we describe the dynamics of microglia-Pax6+ cell associations and interactions after surgical or pharmacological manipulation. In adult rats, the superior cervical ganglia (SCG) were exposed, and either bilaterally excised (SCGx) or decentralized (SCGd). In the SCGx PGs, the density of Iba1+ microglia increased after surgery and returned to sham baseline levels 13 days later. Pineal microglia also responded to SCGd, a more subtle denervation. The number of clustered Iba1+ /PCNA+ /ED1+ microglia was higher 4 days after both surgeries compared to the sham-operated group. However, the number of Pax6+ /PCNA- cells and the percentage of Pax6+ cells contacted by and/or phagocytosed by microglia increased significantly only after SCGx. Separate groups of rats were treated with either bacterial lipopolysaccharides (LPS) or doxycycline (DOX) to activate or inhibit pineal microglia, respectively. Peripheral LPS administration caused an increase in the number of clustered Iba1+ /PCNA+ /ED1+ microglial cells, and in the percentage of Pax6+ cells associated with and/or engulfed by microglia. In the LPS-treated PGs, we also noted an increase in the number of PCNA+ cells that were Iba1- within the microglial cell clusters. The density of Pax6+ cells did not change after LPS treatment. DOX administration did not influence the parameters analyzed. These data suggest that pineal microglia are highly receptive cells capable of rapidly responding in a differential manner to surgical and pharmacological stimuli.
Keywords: ED1 (RRID: AB_566872); Iba1 (RRID: AB_2224402; RRID: AB_839504); PCNA (RRID: AB_95106); Pax6 (RRID: AB_1566562; RRID: AB_2565003; RRID: AB_291612); Tuj1 (RRID: AB_10063408; RRID: AB_2313773); bacterial lipopolysaccharides; decentralization; differential responses; ganglionectomy; microglia; pineal gland.
© 2018 Wiley Periodicals, Inc.
Figures













References
-
- Ajmone-Cat MA, Nicolini A, & Minghetti L (2003). Prolonged exposure of microglia to lipopolysaccharide modifies the intracellular signaling pathways and selectively promotes prostaglandin E2 synthesis. J Neurochem, 87(5), 1193–1203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

