Olanzapine for the prevention and treatment of cancer-related nausea and vomiting in adults
- PMID: 30246876
- PMCID: PMC6513437
- DOI: 10.1002/14651858.CD012555.pub2
Olanzapine for the prevention and treatment of cancer-related nausea and vomiting in adults
Abstract
Background: Olanzapine as an antiemetic represents a new use of an antipsychotic drug. People with cancer may experience nausea and vomiting whilst receiving chemotherapy or radiotherapy, or whilst in the palliative phase of illness.
Objectives: To assess the efficacy and safety of olanzapine when used as an antiemetic in the prevention and treatment of nausea and vomiting related to cancer in adults.
Search methods: We searched CENTRAL, MEDLINE and Embase for published data on 20th September 2017, as well as ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform for unpublished trials. We checked reference lists, and contacted experts in the field and study authors.
Selection criteria: We included randomised controlled trials (RCTs) of olanzapine versus any comparator with or without adjunct therapies for the prevention or treatment, or both, of nausea or vomiting in people with cancer aged 18 years or older, in any setting, of any duration, with at least 10 participants per treatment arm.
Data collection and analysis: We used standard Cochrane methodology. We used GRADE to assess quality of evidence for each main outcome. We extracted data for absence of nausea or vomiting and frequency of serious adverse events as primary outcomes. We extracted data for patient perception of treatment, other adverse events, somnolence and fatigue, attrition, nausea or vomiting severity, breakthrough nausea and vomiting, rescue antiemetic use, and nausea and vomiting as secondary outcomes at specified time points.
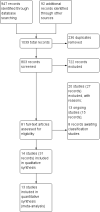
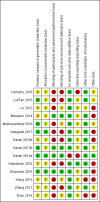
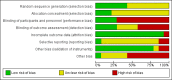
Main results: We included 14 RCTs (1917 participants) from high-, middle- and low-income countries, representing over 24 different cancers. Thirteen studies were in chemotherapy-induced nausea and vomiting. Oral olanzapine was administered during highly emetogenic (HEC) or moderately emetogenic (MEC) chemotherapy (12 studies); chemoradiotherapy (one study); or palliation (one study). Eight studies await classification and 13 are ongoing.The main comparison was olanzapine versus placebo/no treatment. Other comparisons were olanzapine versus NK1 antagonist, prokinetic, 5-HT3 antagonist or dexamethasone.We assessed all but one study as having one or more domains that were at high risk of bias. Eight RCTs with fewer than 50 participants per treatment arm, and 10 RCTs with issues related to blinding, were at high risk of bias. We downgraded GRADE assessments due to imprecision, inconsistency and study limitations.Olanzapine versus placebo/no treatmentPrimary outcomesOlanzapine probably doubles the likelihood of no nausea or vomiting during chemotherapy from 25% to 50% (risk ratio (RR) 1.98, 95% confidence interval (CI) 1.59 to 2.47; 561 participants; 3 studies; solid tumours; HEC or MEC therapy; moderate-quality evidence) when added to standard therapy. Number needed to treat for additional beneficial outcome (NNTB) was 5 (95% CI 3.3 - 6.6).It is uncertain if olanzapine increases the risk of serious adverse events (absolute risk difference 0.7% more, 95% CI 0.2 to 5.2) (RR 2.46, 95% CI 0.48 to 12.55; 7 studies, 889 participants, low-quality evidence).Secondary outcomesFour studies reported patient perception of treatment. One study (48 participants) reported no difference in patient preference. Four reported quality of life but data were insufficient for meta-analysis.Olanzapine may increase other adverse events (RR 1.71, 95% CI 0.99 to 2.96; 332 participants; 4 studies; low-quality evidence) and probably increases somnolence and fatigue compared to no treatment or placebo (RR 2.33, 95% CI 1.30 to 4.18; anticipated absolute risk 8.2% more, 95% CI 1.9 to 18.8; 464 participants; 5 studies; moderate-quality evidence). Olanzapine probably does not affect all-cause attrition (RR 0.99, 95% CI 0.57 to 1.73; 943 participants; 8 studies; I² = 0%). We are uncertain if olanzapine increases attrition due to adverse events (RR 3.00, 95% CI 0.13 to 70.16; 422 participants; 6 studies). No participants withdrew due to lack of efficacy.We are uncertain if olanzapine reduces breakthrough nausea and vomiting (RR 0.38, 95% CI 0.10 to 1.47; 501 participants; 2 studies; I² = 54%) compared to placebo or no treatment. No studies reported 50% reduction in severity of nausea or vomiting, use of rescue antiemetics, or attrition.We are uncertain of olanzapine's efficacy in reducing acute nausea or vomiting. Olanzapine probably reduces delayed nausea (RR 1.71, 95% CI 1.40 to 2.09; 585 participants; 3 studies) and vomiting (RR 1.28, 95% CI 1.14 to 1.42; 702 participants; 5 studies).Subgroup analysis: 5 mg versus 10 mgPlanned subgroup analyses found that it is unclear if 5 mg is as effective an antiemetic as 10 mg. There is insufficient evidence to exclude the possibility that 5 mg may confer a lower risk of somnolence and fatigue than 10 mg.Other comparisonsOne study (20 participants) compared olanzapine versus NK1 antagonists. We observed no difference in any reported outcomes.One study (112 participants) compared olanzapine versus a prokinetic (metoclopramide), reporting that olanzapine may increase freedom from overall nausea (RR 2.95, 95% CI 1.73 to 5.02) and overall vomiting (RR 3.03, 95% CI 1.78 to 5.14).One study (62 participants) examined olanzapine versus 5-HT3 antagonists, reporting olanzapine may increase the likelihood of 50% or greater reduction in nausea or vomiting at 48 hours (RR 1.82, 95% CI 1.11 to 2.97) and 24 hours (RR 1.36, 95% CI 0.80 to 2.34).One study (229 participants) compared olanzapine versus dexamethasone, reporting that olanzapine may reduce overall nausea (RR 1.73, 95% CI 1.37 to 2.18), overall vomiting (RR 1.27, 95% CI 1.10 to 1.48), delayed nausea (RR 1.66, 95% CI 1.33 to 2.08) and delayed vomiting (RR 1.25, 95% CI 1.07 to 1.45).
Authors' conclusions: There is moderate-quality evidence that oral olanzapine probably increases the likelihood of not being nauseous or vomiting during chemotherapy from 25% to 50% in adults with solid tumours, in addition to standard therapy, compared to placebo or no treatment. There is uncertainty whether it increases serious adverse events. It may increase the likelihood of other adverse events, probably increasing somnolence and fatigue. There is uncertainty about relative benefits and harms of 5 mg versus 10 mg.We identified only RCTs describing oral administration. The findings of this review cannot be extrapolated to provide evidence about the efficacy and safety of any injectable form (intravenous, intramuscular or subcutaneous) of olanzapine.
Conflict of interest statement
AS: none known. AS is a specialist trainee Palliative Medicine physician and manages patients with advanced life‐threatening illnesses, including cancer.
KN: none known. KN is a specialist Palliative Medicine Consultant physician and manages patients with advanced life‐threatening illnesses, including cancer.
EP: none known.
KH: none known.
LW: none known. LW is a retired GP and Senior Cochrane UK fellow.
MB: none known. MB is a specialist Ear, Nose and Throat Consultant surgeon and manages patients with illnesses affecting the ear, nose and throat, including cancer. Professor MB is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
BW: none known. BW is a specialist Palliative Medicine Consultant physician and manages patients with advanced life‐threatening illnesses, including cancer. BW is also National Clinical Director for End of Life Care but she has no involvement in commissioning pharmaceutical agents.
Figures
Update of
- doi: 10.1002/14651858.CD012555
Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Antiemetics for adults for prevention of nausea and vomiting caused by moderately or highly emetogenic chemotherapy: a network meta-analysis.Cochrane Database Syst Rev. 2021 Nov 16;11(11):CD012775. doi: 10.1002/14651858.CD012775.pub2. Cochrane Database Syst Rev. 2021. PMID: 34784425 Free PMC article.
-
Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy.Cochrane Database Syst Rev. 2015 Nov 12;2015(11):CD009464. doi: 10.1002/14651858.CD009464.pub2. Cochrane Database Syst Rev. 2015. PMID: 26561338 Free PMC article.
-
Interventions for infantile haemangiomas of the skin.Cochrane Database Syst Rev. 2018 Apr 18;4(4):CD006545. doi: 10.1002/14651858.CD006545.pub3. Cochrane Database Syst Rev. 2018. PMID: 29667726 Free PMC article.
-
Treatments for seizures in catamenial (menstrual-related) epilepsy.Cochrane Database Syst Rev. 2021 Sep 16;9(9):CD013225. doi: 10.1002/14651858.CD013225.pub3. Cochrane Database Syst Rev. 2021. PMID: 34528245 Free PMC article.
Cited by
-
Olanzapine treatment effectively relieves breakthrough chemotherapy-induced nausea and vomiting: a real-world experience.J Pharm Health Care Sci. 2023 Aug 1;9(1):24. doi: 10.1186/s40780-023-00293-y. J Pharm Health Care Sci. 2023. PMID: 37525281 Free PMC article.
-
Olanzapine for the Prevention and Treatment of Chemotherapy-Induced Nausea and Vomiting: A Review to Identify the Best Way to Administer the Drug.Curr Oncol. 2022 Oct 31;29(11):8235-8243. doi: 10.3390/curroncol29110650. Curr Oncol. 2022. PMID: 36354710 Free PMC article. Review.
-
Oral cannabinoid for the prophylaxis of chemotherapy-induced nausea and vomiting-a systematic review and meta-analysis.Support Care Cancer. 2020 May;28(5):2095-2103. doi: 10.1007/s00520-019-05280-4. Epub 2020 Jan 8. Support Care Cancer. 2020. PMID: 31916006
-
Mechanisms of Nausea and Vomiting: Current Knowledge and Recent Advances in Intracellular Emetic Signaling Systems.Int J Mol Sci. 2021 May 28;22(11):5797. doi: 10.3390/ijms22115797. Int J Mol Sci. 2021. PMID: 34071460 Free PMC article. Review.
-
Trends in antipsychotic prescribing among long-term care residents receiving hospice care.J Am Geriatr Soc. 2021 Aug;69(8):2152-2162. doi: 10.1111/jgs.17172. Epub 2021 Apr 9. J Am Geriatr Soc. 2021. PMID: 33837537 Free PMC article.
References
References to studies included in this review
Clemons 2016 {published data only}
-
- Clemons M, Bouganim N, Smith S, Mazzarello S, Vandermeer L, Segal R, et al. Risk model‐guided antiemetic prophylaxis vs physician's choice in patients receiving chemotherapy for early‐stage breast cancer: a randomized clinical trial. JAMA Oncology 2016;2(2):225‐31. - PubMed
-
- Dranitsaris G, Mazzarello S, Smith S, Vandermeer L, Bouganim N, Clemons M. Measuring the impact of guideline‐based antiemetic therapy on nausea and vomiting control in breast cancer patients with multiple risk factors. Supportive Care in Cancer 2016;24(4):1563‐9. - PubMed
Liu/Tan 2015 {published data only}
-
- Liu J, Tan L, Zhang H, Li H, Liu X, Yan Z, et al. QoL evaluation of olanzapine for chemotherapy‐induced nausea and vomiting comparing with 5‐HT3 receptor antagonist. European Journal of Cancer Care 2015;24(3):436‐43. - PubMed
Lu 2013 {published data only}
-
- Lu Y L, Liu W, Du Y J, Feng L, Wang Y D, Wang L. Antiemetic effect of low dose olanzapine in solid tumor chemotherapy. Chinese Journal of Cancer Prevention and Treatment 2013;20(7):544‐54.
Mizukami 2014 {published data only}
-
- Mizukami N, Yamauchi M, Koike K, Watanabe A, Ichihara K, Masumori N, et al. Olanzapine for the prevention of chemotherapy‐induced nausea and vomiting in patients receiving highly or moderately emetogenic chemotherapy: a randomized, double‐blind, placebo‐controlled study. Journal of Pain and Symptom Management 2014;47(3):542‐50. - PubMed
Mukhopadhyay 2016 {published and unpublished data}
-
- Mukhopadhyay S, Kwatra G, Alice K, Badyal D. Role of olanzapine in chemotherapy‐induced nausea and vomiting on platinum‐based chemotherapy patients: a randomized controlled study. Supportive Care in Cancer 2017;25(1):145‐54. - PubMed
-
- Mukhopadhyay S, Kwatra G, Alice P, Badyal D. Role of olanzapine in chemotherapy‐induced nausea and vomiting on platinum‐based chemotherapy patients: a randomized controlled study. Supportive Care in Cancer 2016;25:1‐10. - PubMed
-
- Mukhopadhyay S, Kwatra G, Badyal D, Kingsley P. Olanzapine, concurrent chemo‐radiation patients and chemotherapy induced nausea and vomiting: the surprise continues. Supportive Care in Cancer 2014;22(1 suppl. 1):S99.
-
- Mukhopadhyay S, Kwatra G, Jeyaraj P, Badyal D. Role of olanzapine in chemotherapy induced nausea and vomiting on platinum based chemotherapy patients; a randomized controlled study. Supportive Care in Cancer 2015;23(1 suppl. 1):S126‐7. - PubMed
-
- Mukhopadhyay S, Kwatra G, Kingsley P, Badyal D, Mukhopadhyay B. Olanzapine, an effective and affordable adjuvant in prophylaxis for chemotherapy induced nausea and vomiting on platinum based chemotherapy patients. Clinical Therapeutics 2015;1:e108.
Nakagaki 2017 {published and unpublished data}
-
- Nakagaki M, Barras M, Curley C, Butler J, Kennedy G. A randomized trial of olanzapine and palonosetron versus infused ondansetron for the treatment of chemotherapy induced nausea and vomiting in patients undergoing hematopoietic stem cell transplantation. Blood 2015;126(23):1910. - PubMed
-
- Nakagaki M, Barras M, Curley C, Butler J, Kennedy G. A randomized trial of olanzapine versus palonosetron versus infused ondansetron for the treatment of breakthrough chemotherapy‐induced nausea and vomiting in patients undergoing hematopoietic stem cell transplantation. Supportive Care in Cancer 2017;25(2):607‐13. - PubMed
Navari 2010b {published data only}
-
- Navari R. Treatment of cancer related anorexia with megestrol acetate and olanzapine. Psycho‐Oncology 2009;18:S47‐8. - PubMed
-
- Navari R, Brenner M. Treatment of cancer related anorexia with olanzapine and megestrol acetate. Supportive Care in Cancer 2009;17(7):971. - PubMed
-
- Navari R, Brenner M. Treatment of cancer‐related anorexia with olanzapine and megestrol acetate. Journal of Clinical Oncology 2008;26(15 suppl):9576. - PubMed
-
- Navari R, Brenner M. Treatment of cancer‐related anorexia with olanzapine and megestrol acetate: a randomized trial. Supportive Care in Cancer 2010; Vol. 18, issue 8:951‐6. - PubMed
Navari 2013b {published data only}
-
- Navari R, Nagy C, Gray S. Olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy‐induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy. Supportive Care in Cancer 2012;20:S47. - PubMed
-
- Navari R, Nagy C, Gray S. The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy‐induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy. Journal of Clinical Oncology 2012;30(15 suppl 1):‐. - PubMed
-
- Navari R, Nagy C, Gray S. The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy‐induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Supportive Care in Cancer 2013; Vol. 21, issue 6:1655‐63. - PubMed
Navari 2016a {published data only}
-
- Ahlborn M. Olanzapine: extension of preventive options for nausea and vomiting after highly emetogenic chemotherapy [Olanzapin: erweiterung der praventionsmoglichkeiten von ubelkeit und erbrechen nach hoch emetogener]. Chemotherapie Onkologe 2016;22(12):992‐3.
-
- Mammatas L, Konings I. Olanzapine prevents nausea and vomiting from chemotherapy [Olanzapine voorkomt misselijkheid en braken bij chemotherapie]. Nederlands Tijdschrift voor Geneeskunde 2016;160(41):43.
-
- Navari R, Qin R, Ruddy K, Liu H, Powell S, Bajaj M, et al. Olanzapine for the prevention of chemotherapy‐induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC): Alliance A221301, a randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Oncology 2015;33((29 suppl. 1)):176.
Nikbakhsh 2016 {published data only}
Shumway 2015 {published data only}
-
- Shumway N, Terrazzino S, Jones C. A randomized pilot study comparing aprepitant to olanzapine for treatment of chemotherapy‐induced nausea and vomiting [abstract no. 9633]. Journal of Clinical Oncology 2009;27((15S Part I)):516.
-
- Shumway N, Terrazzino S, Jones C. A randomized pilot study comparing olanzapine (Zyprexa) to aprepitant (Emend) for treatment of chemotherapy induced nausea and vomiting. Journal of Pain Management 2015;8(3):233‐41.
Wang 2015 {published data only}
-
- Wang X, Wang L, Wang H, Zhang H. Effectiveness of olanzapine combined with ondansetron in the prevention of chemotherapy‐induced nausea and vomiting of non‐small cell lung cancer. Cell Biochemistry and Biophysics 2015;72:471‐3. - PubMed
Zhang 2017 {unpublished data only}
-
- Zhang D. Aprepitant, olanzapine, palonosetron and dexamethasone for the prevention of chemotherapy‐induced nausea and vomiting (AOPDPCINV). clinicaltrials.gov/ct2/show/NCT02484911 (accessed 28 September 2017).
Zhao 2014 {published data only}
-
- Zhao J, Li X, Jinghua G, Li Y, Li J, Shi Y, et al. Clinical observation of olanzapine for prevention of high and moderate emetic risk chemotherapy ‐ induced nausea and vomiting. Modern Oncology. Modern Oncology, 2014; Vol. 22, issue 8:1941‐3.
References to studies excluded from this review
Babu 2016 {published data only}
EUCTR2015‐002294‐38‐DK {unpublished data only}
-
- EUCTR2015‐002294‐38‐DK. DANSAC‐pilot: an open label pilot study to investigate the tolerability and efficacy of olanzapine in patients with advanced cancer not receiving chemotherapy or irradiation. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015‐002294‐38‐DK (accessed 28 September 2017).
Flank 2015a {published data only}
-
- Flank J, Robinson P, Holdsworth M, Portwine C, Gibson P, Phillips R, et al. Guideline for the prevention of breakthrough and treatment of refractory chemotherapy‐induced nausea and vomiting in pediatric cancer patients. Canadian Journal of Hospital Pharmacy 2015;68(1):87‐8.
Flank 2015b {published data only}
-
- Flank J, Thackray J, Nielson D, August A, Schechter T, Alexander S, et al. Olanzapine for treatment and prevention of acute chemotherapy‐induced vomiting (CIV) in children: a retrospective, multi‐centre review. Supportive Care in Cancer 2014;22(1 Suppl. 1):S157. - PubMed
-
- Flank J, Thackray J, Nielson D, August A, Schechter T, Alexander S, et al. Olanzapine for treatment and prevention of acute chemotherapy‐induced vomiting in children: a retrospective, multi‐center review. Pediatric Blood and Cancer 2015;62(3):496‐501. - PubMed
-
- Flank J, Thackray J, Nielson D, August A, Schechter T, Alexander S, et al. Olanzapine for treatment and prevention of acute chemotherapy‐induced vomiting in children: a retrospective, multi‐centre review. Pediatric Blood and Cancer 2014;61:S241. - PubMed
Fountaine 2010 {published data only}
-
- Fountaine R, Taylor A, Mancuso J, Greenway F, Byerley L, Smith S, et al. Increased food intake and energy expenditure following administration of olanzapine to healthy men. Obesity (Silver Spring, Md.) 2010;18(8):1646‐51. - PubMed
Guntsch 2012 {published data only}
-
- Guntsch F, Jahn F, Stein A, Russel J, Timo B, Wass M, et al. COMFORT trial‐comparison of olanzapin and metoclopramid for treatment of breakthrough emesis: first experiences of this trial in progress. Onkologie 2012;35:76.
Hashimoto 2016 {published data only}
-
- Hashimoto H, Yanai T, Nagashima K, Tsuda N, Horinouchi H, Takiguchi T, et al. A double‐blind randomized phase II study of 10 versus 5 mg olanzapine for emesis induced by highly emetogenic chemotherapy with cisplatin. Journal of Clinical Oncology 2016;34:(no pagination).
ISRCTN58624349 {unpublished data only}
-
- ISRCTN58624349. Olanzapine (ZYPREXA) versus haloperidol (Novo‐Peridol) for the relief of nausea and vomiting (N&V) in patients with advanced cancer. www.isrctn.com/ISRCTN58624349?q=&filters=conditionCategory:Signs%20a... (accessed 28 September 2017).
Kwatra 2013 {published data only (unpublished sought but not used)}
-
- Kwatra G, Badyal D K, Alice K, Mukhopadhyay P S. Olanzapine for post‐chemotherapy dysgeusia and anorexia: The unidentified saviour?. Supportive Care in Cancer 2013;21:S79.
Long 2017 {published and unpublished data}
-
- Long C, Knoderer H, Mueller E. A pilot study comparing the addition of olanzapine or aprepitant in an antiemetic regimen for highly emetogenic chemotherapyPediatric Blood and Cancer. American Society of Pediatric Hematology/Oncology, ASPHO 2015;62:S104.
-
- NCT02097823. Pilot study of olanzapine and aprepitant to prevent nausea and vomiting in children receiving chemotherapy NCT02097823. www.clinicaltrials.gov (accessed 28 September 2017).
Mukhopadhyay 2012 {published and unpublished data}
-
- Mukhopadhyay S, Kwatra G, Badyal D K, Alice K. Anorexia in concurrent chemoradiation patients: Can olanzapine calm down the monster?. Indian Journal of Physiology and Pharmacology 2012;56(5 SUPPL. 1):219‐20.
Nakashima 2015 {unpublished data only}
-
- Nakashima K, Murakami H. A phase II study of aprepitant, palonosetron, dexamethasone and olanzapine for the prevention of cisplatin‐based hemotherapy‐induced nausea and vomiting for thoracic malignancy. upload.umin.ac.jp/cgi‐open‐bin/ctr/ctr.cgi?function=brows&action=bro... 2015.
Navari 2009a {published data only (unpublished sought but not used)}
-
- Navari R, Gray S. Treatment of chemotherapy‐induced breakthrough nausea and vomiting. Journal of Clinical Oncology 2009;1(27(supp)):e20536.
Navari 2010a {published data only (unpublished sought but not used)}
-
- Navari R, Gray S. Treatment of chemotherapy‐induced breakthrough nausea and vomiting. Psycho‐oncology 2010;19(supp):S1‐102.
Navari 2011 {published data only}
-
- Navari R, Gray S, Kerr A. Olanzapine versus aprepitant for the prevention of chemotherapy induced nausea and vomiting: a randomized Phase III trial. Psycho‐oncology 2012;21:111‐2. - PubMed
-
- Navari R, Gray S, Kerr A. Olanzapine versus aprepitant for the prevention of chemotherapy‐induced nausea and vomiting (CINV): a randomized phase III trial. Journal of Clinical Oncology 2010;28((15 SUPPL. 1)):‐. - PubMed
-
- Navari R, Gray S, Kerr A. Olanzapine versus aprepitant for the prevention of chemotherapy‐induced nausea and vomiting: a randomized phase III trial. The Journal of Supportive Oncology 2011; Vol. 9, issue 5:188‐95. - PubMed
-
- Navari R, Gray S, Kerr, A. Olanzapine versus aprepitant for the prevention of chemotherapy induced nausea and vomiting (CINV): a randomized trial. Supportive Care in Cancer 2010;18:S81‐2. - PubMed
Navari 2016b {published data only}
-
- Navari R, Nagy C. Olanzapine versus fosaprepitant for the prevention of nausea and vomiting in patients receiving concurrent chemoradiation treatment. Journal of Clinical Oncology 2015;33(15 suppl. 1):‐.
-
- Navari R, Nagy C, Le‐Rademacher J, Loprinzi C. Olanzapine versus fosaprepitant for the prevention of concurrent chemotherapy radiotherapy‐induced nausea and vomiting. The Journal of Community and Supportive Oncology 2016;14(4):141‐7. - PubMed
NCT00124930 {unpublished data only}
-
- NCT00124930. Study comparing olanzapine with haloperidol for the relief of nausea and vomiting in patients with advanced cancer. clinicaltrials.gov/ct2/show/NCT00124930 (first received 29 July 2005).
NCT01148264 {unpublished data only}
-
- NCT01148264. Comparison of olanzapine and metoclopramide for treatment of breakthrough emesis (COMFORT). www.clinicaltrials.gov (accessed 28 September 2017).
Slimano 2016 {published and unpublished data}
-
- Slimano F, Netzer F, Borget I, Lemare F, Besse B. Is the addition of olanzapine effective in restoration of CINV prevention in non‐responders to standard antiemetic therapy? A retrospective controlled study. International Journal of Clinical Pharmacy 2016;38(6):491.
Yanai 2015 {unpublished data only}
-
- Yanai T, Yamamoto N. A double‐blind randomized phase II study of olanzapine 10mg versus 5mg for highly emetogenic chemotherapy‐induced nausea and vomiting. rctportal.niph.go.jp/en/detail?trial_id=UMIN000014214 2015.
References to studies awaiting assessment
Chasick 2012 {published data only}
-
- Chasick A, DeFreitas E, Edwards M. Olanzapine for the prevention of chemotherapy‐induced nausea and vomiting in patients receiving highly or moderately emetogenic antineoplastic chemotherapy. Journal of the American Pharmacists Association 2012;52(5):681.
CTRCC‐14004093 {published data only (unpublished sought but not used)}
-
- ChiCTR‐TTRCC‐14004093. Olanzapine versus aprepitant for the prevention of high dose cisplatin‐induced nausea and vomiting: a randomized phase III trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR‐TTRCC‐14004093 (accessed 28 September 2017).
Jeon 2017 {published data only}
-
- Jeon S, Han H, Bae W, Park M, Lee S, Go S, et al. A randomized, double‐blind, placebo‐controlled study of the safety and efficacy of olanzapine for the prevention of chemotherapy‐induced nausea and vomiting in patients receiving moderately emetogenic chemotherapy. Journal of Clinical Oncology 2017;35(15 Supplement 1):(no pagination).
Mao 2011 {published data only (unpublished sought but not used)}
-
- Mao W‐K, Peng L. Clinical observation of olanzepine combined with granisterom and hexadecadrol prevent nausea vomit induced by chemoradiotherapy. Chinese Journal of Medicinal Guide 2011;13(3):452‐4.
Meng 2016 {published data only (unpublished sought but not used)}
-
- Meng Q, Chen G, Guo P. Olanzapine combined with normal antiemetic drugs in patients on solid tumor chemotherapy: antiemetic effect and impact on quality of life. World Chinese Journal of Digestology 2016;24(7):1117‐23.
Mukesh 2017 {published data only (unpublished sought but not used)}
-
- Mukesh S, Sathya M, Akshay J. Olanzapine versus aprepitant in the prevention of chemotherapy induced nausea and vomiting (CINV) in breast cancer patients. Journal of Clinical Oncology 2017;35(15 Supplement 1):‐.
Nguyen 2017 {published data only (unpublished sought but not used)}
-
- Nguyen N, Pham V, Nguyen T, Tran T. Olanzapine and omeprazole combination is simple, safe and effective for delayed nausea and vomiting control in adjuvant chemotherapy for early stage breast cancer. Supportive Care in Cancer 2017;25(2 Supplement 1):S72.
Wang 2012 {published data only}
-
- Wang, Wang. Effectiveness of olanzapine in prevention of chemotherapy induced nausea and vomiting. Clin J Clin (full journal title not available) 2012;6:7406‐7.
References to ongoing studies
Abe 2017 {unpublished data only}
-
- Abe M, Yanai T, Nakashima K, Hirashima Y, Murakami H, Hamauchi S, et al. A randomized, double‐blind, placebo‐controlled phase iii study evaluating olanzapine 5mg combined with standard antiemetic therapy for the prevention of CINV in patients receiving cisplatin‐based chemotherapy. Supportive Care in Cancer. Conference: 2017 International MASCC/ISOO Symposium 2017;25((2 Supplement 1)):S632017.
ChiCTR‐TTRCC‐14004093 {unpublished data only}
-
- ChiCTR‐TTRCC‐14004093. Olanzapine versus aprepitant for the prevention of high‐dose cisplatin‐induced nausea and vomiting: a randomised phase III trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR‐TTRCC‐14004093 (accessed 28 September 2017) (first received 08 January 2014).
Hashimoto 2017 {published data only (unpublished sought but not used)}
-
- Hashimoto H, Abe M. A randomized, double‐blind, placebo‐controlled phase III trial evaluating olanzapine 5 mg combined with standard antiemetic therapy for the prevention of chemotherapy‐induced nausea and vomiting in patients receiving cisplatin‐based highly emetogenic chemotherapy: J‐SUPPORT 1604. rctportal.niph.go.jp/en/detail?trial_id=UMIN000024676 2017;‐:‐. - PubMed
-
- Hashimoto H, Iwasa S, Abe M, Yanai T, Zenda S, Yamaguchi T, et al. 1607TiP ‐ J‐FORCE study: a randomized, double‐blind, placebo‐controlled phase III study evaluating olanzapine (5 mg) combined with standard antiemetic therapy. Annals of Oncology 2017;28(suppl_5):v543‐67.
JPRN‐UMIN000010317 {unpublished data only}
-
- JPRN‐UMIN000010317. Efficacy of olanzapine for relief of nausea with incomplete bowel obstruction in advanced cancer patient: pragmatic randomized controlled trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000010317 (first received 26 March 2013).
Mukhopadhyay 2017a {published data only (unpublished sought but not used)}
-
- Mukhopadhyay S, Dutta P, Bhattacharya B, Banerjee S, Biswas S, Mukhopadhyay‐Samanta B. Low dose vs. standard dose adjuvant olanzapine in chemotherapy induced nausea and vomiting: a prospective, randomized, double blinded, controlled study. Clinical Therapeutics 2017;39(8 Supplement 1):e17.
-
- Mukhopadhyay S, Dutta P, Bhattacharya B, Banerjee S, Biswas S, Samanta B. Comparison of efficacy, safety and sedation with two doses of add on olanzapine in chemotherapy induced nausea and vomiting: a randomized controlled pilot study. Supportive Care in Cancer 2016;24(1 Supplement 1):S248‐9.
-
- Mukhopadhyay S, Dutta P, Bhattacharya B, Das D, Biswas S. Low dose olanzapine in chemotherapy induced nausea and vomiting: a balance game between sedation and nausea?. Supportive Care in Cancer: International MASCC/ISOO Symposium 2017;25(2 Supplement 1):S69.
Nagashima et al 2015 {published data only}
-
- Nagashima K, Iwasa S, Yanai T, Hashimoto H, Suzuki K, Ohyanagi F, et al. A double‐blind randomized phase II study of olanzapine 10 mg versus 5 mg for emesis induced by highly emetogenic chemotherapy. Japanese Journal of Clinical Oncology 2015;45(2):229‐31. - PubMed
NCT02290470 {unpublished data only}
-
- NCT02290470. Olanzapine against delayed nausea and vomiting in women receiving carboplatin plus paclitaxel. clinicaltrials.gov/ct2/show/NCT02290470 (first received 14 November 2014).
NCT02400866 {unpublished data only}
-
- NCT02400866. A randomized study of olanzapine for the prevention of CINV in patients receiving moderately emetogenic chemotherapy. clinicaltrials.gov/ct2/show/NCT02400866 (first received 27 March 2015).
NCT02635984 {unpublished data only}
-
- NCT02635984. Study of FOND versus FOND+O for the prevention of CIV in hematology patients receiving highly emetogenic chemotherapy regimens [Randomized, placebo controlled study of FOND (fosaprepitant, ondansetron, dexamethasone) versus FOND+O (FOND plus olanzapine) for the prevention of chemotherapy induced nausea and vomiting in hematology patients receiving highly emetogenic chemotherapy regimens]. clinicaltrials.gov/ct2/show/NCT02635984 (first received 21 December 2015).
NCT02939287 {unpublished data only}
-
- NCT02939287. Aprepitant versus olanzapine with high dose melphalan [Aprepitant versus olanzapine for prevention of acute and delayed nausea and vomiting associated with high dose melphalan and BEAM in autologous stem dell transplant patients]. clinicaltrials.gov/ct2/show/NCT02939287 (first received 20 October 2016).
NCT02970643 {unpublished data only}
-
- NCT02970643. Proof‐of‐concept trial of palonosetron and olanzapine without dexamethasone for the prevention of CIN [Proof‐of‐concept trial of palonosetron and olanzapine without dexamethasone for the prevention of chemotherapy‐induced nausea and vomiting]. clinicaltrials.gov/ct2/show/NCT02970643 (first received 22 November 2016).
NCT03079219 {unpublished data only}
-
- NCT03079219. Olanzapine for the prevention of chemotherapy‐induced nausea and vomiting in Chinese breast cancer patients [A randomized study to determine the efficacy and tolerability of olanzapine for the prevention of chemotherapy‐induced nausea and vomiting in Chinese breast cancer patients]. clinicaltrials.gov/ct2/show/NCT03079219 (first received 14 March 2017).
NCT03137121 {unpublished data only}
-
- NCT03137121. Olanzapine for the treatment of chronic nausea and/or vomiting in advanced cancer patients [Olanzapine for the treatment of chronic nausea and/or vomiting, unrelated to chemotherapy or radiation, in advanced cancer patient ‐ a pilot, dose‐finding trial]. clinicaltrials.gov/ct2/show/NCT03137121 (first received 2 May 2017).
Additional references
Anonymous 2017
-
- Anonymous. Zyprexa 2.5 mg, 5 mg, 7.5 mg, 10 mg, 15 mg, and 20 mg coated tablets. Zyprexa Velotab 5 mg, 10 mg, 15 mg, and 20 mg orodispersible tablets. www.medicines.org.uk/emc/medicine/614 (accessed 29 September 2017).
BNF
-
- British National Formulary. www.evidence.nhs.uk/formulary/bnf/current/4‐central‐nervous‐system/42‐dr... (accessed 28 September 2017).
Burke 2011
-
- Burke T, Wisniewski T, Ernst F. Resource utilization and costs associated with chemotherapy‐induced nausea and vomiting (CINV) following highly or moderately emetogenic chemotherapy administered in the US outpatient hospital setting. Supportive Care in Cancer 2011;19(1):131‐40. - PubMed
Chanthawong 2017
-
- Chanthawong S, Lim Y H, Subongkot S, Chan A, Andalusia R, Bustamam R S, et al. Cost‐effectiveness analysis of olanzapine‐containing antiemetic therapy for managing highly emetogenic chemotherapy in south east Asia: a multinational study. Supportive Care in Cancer 2017;25(2 Supplement 1):S64‐5. - PubMed
Chelkeba 2017
Chiu 2016a
-
- Chiu L, Chow R, Popovic M, Navari R, Shumway N, Chiu N, et al. Efficacy of olanzapine for the prophylaxis and rescue of chemotherapy‐induced nausea and vomiting (CINV): a systematic review and meta‐analysis. Supportive Care in Cancer 2016;24(5):2381‐92. - PubMed
Chow 2016
-
- Chow R, Chiu L, Navari R, Passik S, Chiu N, Popovic M, et al. Efficacy and safety of olanzapine for the prophylaxis of chemotherapy‐induced nausea and vomiting (CINV) as reported in phase I and II studies: a systematic review. Supportive Care in Cancer 2016;24(2):1001‐8. - PubMed
Cohen 2007
-
- Cohen L, Moor C, Eisenberg P, Ming E, Hu H. Chemotherapy‐induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Supportive Care in Cancer 2007;15(5):497‐503. - PubMed
Covidence [Computer program]
-
- Veritas Health Innovation. Covidence. Version 30 September 2017. Melboune, Australia: Veritas Health Innovation, 2017.
Cox 2015
Deeks 2017
-
- Deeks J J, Higgins J P, Altman D G (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins J P T, Churchill R, Chandler J, Cumpston M S (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Egger 1997
FDA
-
- US Food, Drug Administration (FDA). ZYPREXA® olanzapine tablets ZYPREXA® ZYDIS® olanzapine orally disintegrating tablets ZYPREXA® intramuscular olanzapine for Injection. www.fda.gov/ohrms/dockets/ac/08/briefing/2008‐4399b1‐05%20(Zyprexa%20(ol... accessed 23 October 2017.
Glare 2011
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
GraphPad [Computer program]
-
- GraphPad Software Inc. GraphPad. Version accessed 26 October 2017. GraphPad Software Inc, 2017.
Guyatt 2011
-
- Guyatt G H, Oxman A D, Schunemann H J, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64:380‐2. - PubMed
Higgins 2003
Higgins 2017
-
- Higgins J P, Altman D G, Sterne J A C (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins J P T, Churchill R, Chandler J, Cumpston M S (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hjermstad 2013
Hocking 2014
-
- Hocking C, Kichenadasse G. Olanzapine for chemotherapy‐induced nausea and vomiting: a systematic review. Supportive Care in Cancer 2014;22(4):1143‐51. - PubMed
Jackson 2003
-
- Jackson W, Tavernier L. Olanzapine for intractable nausea in palliative care patients. Journal of Palliative Medicine 2003;6(2):251‐5. - PubMed
Kishi 2015
-
- Kishi T, Hirota T, Matsunaga S, Iwata N. Antipsychotic medications for the treatment of delirium: a systematic review and meta‐analysis of randomised controlled trials. Journal of Neurology, Neurosurgery, and Psychiatry 2016;87(7):767‐74. - PubMed
Kris 2011
-
- Kris M. Impact of CINV. Clinical Advances in Hematology and Oncology 2011;9(1):9‐13. - PubMed
Kumar 2017
-
- Kumar R, Singh N, Thekkekara R, Singh S, Harrington S, Shah M. Efficacy of olanzapine for prevention of chemotherapy‐induced nausea and vomiting: A systematic review and meta‐analysis. Journal of Clinical Oncology. Conference 2017;35(15 Supplement 1):‐.
Martel 2016
-
- Martel M, Klein L, Rivard R, Colev J. A large retrospective cohort of patients receiving intravenous olanzapine in the emergency department. Society for Academic Emergency Medicine 2016;23:29‐33. - PubMed
Medicines.org
-
- EMC. Nytol One‐A‐Night. www.medicines.org.uk/emc/medicine/19778 (accessed 1 November 2017).
Medicines.org a
-
- EMC. Zofran Tablets 4 mg and 8 mg. www.medicines.org.uk/emc/medicine/17650/spc (accessed 1 November 2017).
Moher 2009
Murray‐Brown 2015
Navari 2013a
-
- Navari R. Management of chemotherapy‐induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs 2013;73(3):249‐62. - PubMed
Pirl 2000
-
- Pirl W F, Roth A J. Remission of chemotherapy‐induced emesis with concurrent olanzapine treatment: a case report. Psycho‐oncology 2000;9(1):84‐7. - PubMed
Prommer 2012
-
- Prommer E. Olanzapine: palliative medicine update. American Journal of Hospice & Palliative Medicine 2012;30(1):75‐82. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
-
- Schünemann H J, Oxman A D, Higgins J P, Vist G E, Glasziou P, Guyatt G H. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins J P T, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Schünemann 2017
-
- Schünemann H J, Oxman A D, Higgins J P, Vist G E, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins J P T, Churchill R, Chandler J, Cumpston M S (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Solano 2006
-
- Solano J, Gomes B, Higginson I. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. Journal of Pain and Symptom Management 2006;31(1):58–69. - PubMed
Sommariva 2016
-
- Sommariva S, Pongiglione B, Tarricone R. Impact of chemotherapy‐induced nausea and vomiting on health‐related quality of life and resource utilization: a systematic review. Critical Reviews in Oncology/Hematology 2016;99:13‐36. - PubMed
Teunissen 2007
-
- Teunissen S, Wesker W, Kruitwagen C, Haes H, Voest E, Graeff A. Symptom prevalence in patients with incurable cancer: a systematic review. Journal of Pain and Symptom Management 2007;31(1):94‐104. - PubMed
van der Vorst 2015
Wang 2014
-
- Wang S, Yang Z, Zhang L. Olanzapine for preventing nausea and vomiting induced by moderately and highly emetogenic chemotherapy. Asian Pacific Journal of Cancer Prevention 2014;15(22):9587‐92. - PubMed
Yang 2017
Yoodee 2017
-
- Yoodee J, Permsuwan U, Nimworapan M. Efficacy and safety of olanzapine for the prevention of chemotherapy‐induced nausea and vomiting: A systematic review and meta‐analysis. Critical Reviews in Oncology/Hematology 2017;112:‐. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical