Intramuscular versus intravenous prophylactic oxytocin for the third stage of labour
- PMID: 30246877
- PMCID: PMC6513632
- DOI: 10.1002/14651858.CD009332.pub3
Intramuscular versus intravenous prophylactic oxytocin for the third stage of labour
Update in
-
Intravenous versus intramuscular prophylactic oxytocin for the third stage of labour.Cochrane Database Syst Rev. 2020 Nov 9;11(11):CD009332. doi: 10.1002/14651858.CD009332.pub4. Cochrane Database Syst Rev. 2020. PMID: 33169839 Free PMC article.
Abstract
Background: There is general agreement that oxytocin given either through the intramuscular or intravenous route is effective in reducing postpartum blood loss. However, it is unclear whether the subtle differences between the mode of action of these routes have any effect on maternal and infant outcomes. This is an update of a review first published in 2012.
Objectives: To determine the comparative effectiveness and safety of oxytocin administered intramuscularly or intravenously for prophylactic management of the third stage of labour after vaginal birth.
Search methods: We searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (7 September 2017) and reference lists of retrieved studies.
Selection criteria: Randomised trials comparing intramuscular with intravenous oxytocin for prophylactic management of the third stage of labour after vaginal birth. We excluded quasi-randomised trials.
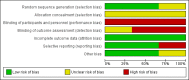
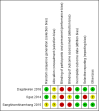
Data collection and analysis: Two review authors independently assessed studies for inclusion and risk of bias, extracted data and checked them for accuracy. We assessed the quality of the evidence using the GRADE approach.
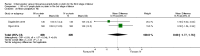
Main results: Three studies with 1306 women are included in the review and compared intramuscular versus intravenous oxytocin administered just after the birth of the anterior shoulder or soon after the birth of the baby. Studies were carried out in hospital settings in Turkey and Thailand and recruited women with singleton, term pregnancies. Overall, the included studies were at moderate risk of bias: none of the studies provided clear information on allocation concealment or attempted to blind staff or women. For GRADE outcomes the quality of the evidence was very low, with downgrading due to study design limitations and imprecision of effect estimates.Only one study reported severe postpartum haemorrhage (blood loss 1000 mL or more) and showed no clear difference between the intramuscular and intravenous oxytocin groups (risk ratio (RR) 0.11, 95% confidence interval (CI) 0.01 to 2.04; 256 women; very low-quality evidence). No woman required hysterectomy in either group in one study (no estimable data, very low-quality evidence), and in another study one woman in each group received a blood transfusion (RR 1.00, 95% CI 0.06 to 15.82; 256 women; very low-quality evidence). Other important outcomes (maternal death, hypotension, maternal dissatisfaction with the intervention and neonatal jaundice) were not reported by any of the included studies. There were no clear differences between groups for other prespecified secondary outcomes reported (postpartum haemorrhage 500 mL or more, use of additional uterotonics, retained placenta or manual removal of the placenta).
Authors' conclusions: Very low-quality evidence indicates no clear difference between the comparative benefits and risks of intramuscular and intravenous oxytocin when given to prevent excessive blood loss after vaginal birth. Appropriately designed randomised trials with adequate sample sizes are needed to assess whether the route of prophylactic oxytocin after vaginal birth affects maternal or infant outcomes. Such studies could be large enough to detect clinically important differences in major side effects that have been reported in observational studies and should also consider the acceptability of the intervention to mothers and providers as important outcomes.
Conflict of interest statement
OT Oladapo: the first version of this review was performed under a contractual Agreement for Performance of Work (APW) between World Health Organization (WHO), and the contact author. No funding was allocated for the preparation of this update. However, the contact author is currently a paid staff member of the WHO.
BO Okusanya: none known
E Abalos: this systematic review was prepared in order to compile the existing evidence for the update of the WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage.
Figures










Update of
-
Intramuscular versus intravenous prophylactic oxytocin for the third stage of labour.Cochrane Database Syst Rev. 2012 Feb 15;(2):CD009332. doi: 10.1002/14651858.CD009332.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2018 Sep 22;9:CD009332. doi: 10.1002/14651858.CD009332.pub3. PMID: 22336865 Updated.
References
References to studies included in this review
-
- Dagdeviren H. Intramuscular versus intravenous prophylactic oxytocin for hemorrhage after vaginal delivery (oxytocin). clinicaltrials.gov/ct2/show/NCT02080104 (first received 1 March 2014). - PubMed
- Dagdeviren H, Cengiz H, Heydarova U, Caypinar SS, Kanawati A, Guven E, et al. Intramuscular versus intravenous prophylactic oxytocin for postpartum hemorrhage after vaginal delivery: a randomized controlled study. Archives of Gynecology and Obstetrics 2016;294(5):911‐6. - PubMed
-
- NCT01954186. When and how to administer oxytocin for active management of third stage of labour. clinicaltrials.gov/show/NCT01954186 (first received 22 September 2013).
- Oguz Orhan E, Dilbaz B, Aksakal SE, Altinbas S, Erkaya S. Prospective randomized trial of oxytocin administration for active management of the third stage of labor. International Journal of Gynecology and Obstetrics 2014;127(2):175‐9. - PubMed
-
- ACTRN12612000624886. A randomised controlled trial of intravenous versus intramuscular oxytocin in the management of third stage of labor. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612000624886 (first received 12 June 2012).
- Sangkomkamhang U, Kruangpatee. A randomized controlled trial of intravenous versus intramuscular oxytocin in the prevention of postpartum hemorrhage during the third stage of labor. Journal of Health Science 2015;24(2):354‐9.
References to studies excluded from this review
-
- Durocher J, Blum J, Sheldon WR, Trussell J, Winikoff B. Does the effect of oxytocin prophylaxis on post‐partum blood loss depend on route of administration?. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S332.
- Sheldon W. Gynuity Health Projects: assessing effectiveness of the AMTSL components. Personal communicationJuly 2011.
References to studies awaiting assessment
-
- ACTRN12617000176369. Correlative assessment of prophylactic oxytocin pharmacokinetics (PK) to maternal body mass index (BMI) following intravenous (IV) and intramuscular (IM) administration, for the prevention of postpartum haemorrhage, at elective caesarean section. An open label, prospective, exploratory, randomised controlled trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12617000176369 (first received 23 January 2017).
-
- NCT02319707. Management of the third stage of labor. clinicaltrials.gov/ct2/show/NCT02319707 (first received 14 December 2014).
-
- Neri‐Mejia M, Pedraza‐Aviles AG. Active management of the third stage of labor: three schemes of oxytocin: randomised clinical trial. Ginecologia y Obstetricia De Mexico 2016;84(5):306‐13. - PubMed
References to ongoing studies
-
- Ashwal E, Hiersch L, Wertheimer A, Krispin E, Aviram A, Dayan DB, et al. The effect of post‐partum oxytocin regimen on hemoglobin decline–a randomized controlled trial. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S197‐S198, Abstract no: 351.
-
- Dzuba I, Durocher J, Dilbaz B, Gelisen O, Ngoc NTN, Montesinos R, et al. Route of administration of oxytocin in prevention of postpartum hemorrhage. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S333.
-
- ISRCTN14718882. A study to compare the effectiveness of intravenous oxytocin with intramuscular oxytocin given at the third stage of labour at preventing bleeding at vaginal birth. isrctn.com/ISRCTN14718882 (first received 14 December 2015).
-
- NCT00200252. Intramuscular versus intravascular oxytocin for the third stage of labour. clinicaltrials.gov/ct2/show/record/NCT00200252 (first received 12 September 2015).
-
- NCT01608958. Intravenous and intramuscular administration of oxytocin in the third stage of labor for prevention of postpartum hemorrhage. clinicaltrials.gov/ct2/show/NCT01608958 (first received 29 May 2012).
Additional references
-
- Blum J, Winikoff B, Raghavan S, Dabash R, Ramadan MC, Dilbaz B, et al. Treatment of post‐partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double‐blind, randomised, non‐inferiority trial. Lancet 2010;375(9710):217‐23. - PubMed
-
- Bolton TJ, Randall K, Yentis SM. Effect of the Confidential Enquiries into Maternal Deaths on the use of syntocinon at caesarean section in the UK. Anaesthesia 2003;58:277‐9. - PubMed
-
- Breathnach F, Geary M. Standard medical therapy. In: B‐Lynch C, Keith LG, Lalonde AB, Karoshi M editor(s). A Textbook of Postpartum Haemorrhage. United Kingdom: Sapiens publishing, 2006:256‐62.
-
- Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Practice & Research Clinical Obstetrics and Gynaecology 2008;22:999–1012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

