Molecular and clinical relationship between live-attenuated Japanese encephalitis vaccination and childhood onset myasthenia gravis
- PMID: 30246904
- PMCID: PMC6175482
- DOI: 10.1002/ana.25267
Molecular and clinical relationship between live-attenuated Japanese encephalitis vaccination and childhood onset myasthenia gravis
Abstract
Objective: The incidence of childhood onset myasthenia gravis (CMG) in China is higher than that in other countries; however, the reasons for this are unclear.
Methods: We investigated the clinical and immunological profiles of CMG, and assessed the potential precipitating factors. For the mouse studies, the possible implication of vaccination in the pathogenesis was explored.
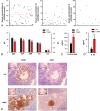
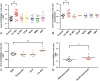
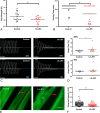
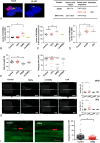
Results: In our retrospective study, 51.22% of the 4,219 cases of myasthenia gravis (MG) were of the childhood onset type. The cohort study uncovered that the pathophysiology of CMG was mediated by immune deviation, rather than through gene mutations or virus infections. The administration of the live-attenuated Japanese encephalitis vaccine (LA-JEV), but not the inactivated vaccine or other vaccines, in mice induced serum acetylcholine receptor (AChR) antibody production, reduced the AChR density at the endplates, and decreased both muscle strength and response to repetitive nerve stimulation. We found a peptide (containing 7 amino acids) of LA-JEV similar to the AChR-α subunit, and immunization with a synthesized protein containing this peptide reproduced the MG-like phenotype in mice.
Interpretation: Our results describe the immunological profile of CMG. Immunization with LA-JEV induced an autoimmune reaction against the AChR through molecular mimicry. These findings might explain the higher occurrence rate of CMG in China, where children are routinely vaccinated with LA-JEV, compared with that in countries, where this vaccination is not as common. Efforts should be made to optimize immunization strategies and reduce the risk for developing autoimmune disorders among children. Ann Neurol 2018;84:386-400.
© 2018 The Authors. Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Figures


References
-
- Zhu WH, Lu JH, Lin J, et al. HLA‐DQA1*03:02/DQB1*03:03:02 is strongly associated with susceptibility to childhood‐onset ocular myasthenia gravis in Southern Han Chinese. J Neuroimmunol 2012;247:81–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

