Polymerizing Like Mussels Do: Toward Synthetic Mussel Foot Proteins and Resistant Glues
- PMID: 30246912
- PMCID: PMC6282983
- DOI: 10.1002/anie.201809587
Polymerizing Like Mussels Do: Toward Synthetic Mussel Foot Proteins and Resistant Glues
Abstract
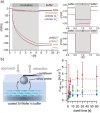
A novel strategy to generate adhesive protein analogues by enzyme-induced polymerization of peptides is reported. Peptide polymerization relies on tyrosinase oxidation of tyrosine residues to Dopaquinones, which rapidly form cysteinyldopa-moieties with free thiols from cysteine residues, thereby linking unimers and generating adhesive polymers. The resulting artificial protein analogues show strong adsorption to different surfaces, even resisting hypersaline conditions. Remarkable adhesion energies of up to 10.9 mJ m-2 are found in single adhesion events and average values are superior to those reported for mussel foot proteins that constitute the gluing interfaces.
Keywords: adhesives; enzyme-induced polymerization; mussel glue; synthetic protein mimics; tyrosinase activation.
© 2018 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- None
-
- Lee H., Lee B. P., Messersmith P. B., Nature 2007, 448, 338–341; - PubMed
-
- Wilke P., Helfricht N., Mark A., Papastavrou G., Faivre D., Börner H. G., J. Am. Chem. Soc. 2014, 136, 12667—12674; - PubMed
-
- Wei Q., Achazi K., Liebe H., Schulz A., Noeske P.-L. M., Grunwald I., Haag R., Angew. Chem. Int. Ed. 2014, 53, 11650–11655; - PubMed
- Angew. Chem. 2014, 126, 11834–11840;
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

