Cost-Effectiveness and Budget Impact of Lumacaftor/Ivacaftor in the Treatment of Cystic Fibrosis
- PMID: 30247102
- PMCID: PMC10397671
- DOI: 10.18553/jmcp.2018.24.10.987
Cost-Effectiveness and Budget Impact of Lumacaftor/Ivacaftor in the Treatment of Cystic Fibrosis
Erratum in
-
Corrections.J Manag Care Spec Pharm. 2019 Feb;25(2):285-286. doi: 10.18553/jmcp.2019.25.2.285. J Manag Care Spec Pharm. 2019. PMID: 30698088 Free PMC article. No abstract available.
Abstract
Background: Cystic fibrosis (CF) is a chronic, progressive, genetic disease affecting more than 30,000 people in the United States and 70,000 people globally. The goals of treatment are to slow disease progression, reduce pulmonary exacerbations, relieve chronic symptoms, and improve the patient's quality of life. Lumacaftor/ivacaftor is a new therapy for CF that has demonstrated good clinical outcomes, including improved absolute percentage predicted forced expiratory volume in 1 second (FEV1%). However, given the high cost of therapy, there is a need to evaluate the overall value of lumacaftor/ivacaftor in CF management.
Objectives: To (a) conduct a cost-effectiveness analysis (CEA) of lumacaftor/ivacaftor to understand the overall effectiveness of the drug compared with its costs and (b) conduct a budget impact analysis (BIA) to understand the potential financial effect of introducing a new drug in a health plan.
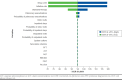
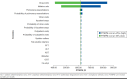
Methods: Two static decision models were developed using Microsoft Excel to evaluate the cost-effectiveness and budget impact of lumacaftor/ivacaftor over a 1-year time frame from a payer perspective. Model inputs included drug costs (wholesale acquisition costs), drug monitoring schedules (package inserts), drug monitoring costs (Centers for Medicare & Medicaid physician fee schedule and published literature), FEV1% predicted and pulmonary exacerbation values (clinical trials), and cost to treat pulmonary exacerbations (published literature). The outcomes in the CEA included total cost of therapy; average cost-effectiveness ratio (ACER), defined as cost per FEV1% predicted; and incremental cost-effectiveness ratio (ICER), defined as the difference in the ratio of cost per FEV1% predicted of lumacaftor/ivacaftor and placebo. Outcomes in the BIA included total budget impact; cost per member per month (PMPM), defined as total budget impact per hypothetical plan population; and cost per treated member per month (PTMPM), defined as total budget impact per target CF population. All costs were adjusted to 2016 dollars, and one-way sensitivity analyses were conducted to test the model robustness given uncertainty in model inputs and study assumptions.
Results: The annual cost of therapy per patient for lumacaftor/ivacaftor was $379,780. The ACER for lumacaftor/ivacaftor was $151,912, while the ICER for lumacaftor/ivacaftor compared with placebo was $95,016 per FEV1% predicted. The annual total budget impact due to the inclusion of lumacaftor/ivacaftor on the health plan formulary was $266,046. The PMPM cost was $0.02 and the PTMPM cost was $6.21.
Conclusions: In patients with CF, lumacaftor/ivacaftor has demonstrated better clinical effectiveness compared with placebo alongside an increased drug acquisition cost. However, the therapy may be a viable alternative to existing standard therapy over a short time horizon. Health care payers, both private and public, need to evaluate the cost-effectiveness and the financial effect when considering expansion of new drug coverage in CF management.
Disclosures: No outside funding supported this study. Covvey and Kamal have received research funding from Novartis Pharmaceuticals. Covvey, Giannetti, and Kamal have received research funding from the College of Psychiatric and Neurologic Pharmacists. Kamal serves as a consultant to the Lynx Group (Cranbury, NJ) and Manticore Consulting Group (Scottsdale, AZ). Mukherjee has nothing to disclose. A related poster abstract was presented at the AMCP Managed Care & Specialty Pharmacy Annual Meeting; March 27-30, 2017; Denver, CO.
Conflict of interest statement
No outside funding supported this study. Covvey and Kamal have received research funding from Novartis Pharmaceuticals. Covvey, Giannetti, and Kamal have received research funding from the College of Psychiatric and Neurologic Pharmacists. Kamal serves as a consultant to the Lynx Group (Cranbury, NJ) and Manticore Consulting Group (Scottsdale, AZ). Mukherjee has nothing to disclose.
A related poster abstract was presented at the AMCP Managed Care & Specialty Pharmacy Annual Meeting; March 27-30, 2017; Denver, CO.
Figures
Similar articles
-
Ivacaftor-tezacaftor-elexacaftor, tezacaftor-ivacaftor and lumacaftor-ivacaftor for treating cystic fibrosis: a systematic review and economic evaluation.Health Technol Assess. 2025 May;29(19):1-111. doi: 10.3310/CPLD8546. Health Technol Assess. 2025. PMID: 40418577 Free PMC article.
-
Cost-effectiveness analysis of lumacaftor and ivacaftor combination for the treatment of patients with cystic fibrosis in the United States.Orphanet J Rare Dis. 2018 Sep 29;13(1):172. doi: 10.1186/s13023-018-0914-3. Orphanet J Rare Dis. 2018. PMID: 30268148 Free PMC article.
-
Forecasting the Long-Term Clinical and Economic Outcomes of Lumacaftor/Ivacaftor in Cystic Fibrosis Patients with Homozygous phe508del Mutation.Value Health. 2017 Dec;20(10):1329-1335. doi: 10.1016/j.jval.2017.06.014. Epub 2017 Aug 1. Value Health. 2017. PMID: 29241892
-
Correctors (specific therapies for class II CFTR mutations) for cystic fibrosis.Cochrane Database Syst Rev. 2018 Aug 2;8(8):CD010966. doi: 10.1002/14651858.CD010966.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 Dec 17;12:CD010966. doi: 10.1002/14651858.CD010966.pub3. PMID: 30070364 Free PMC article. Updated. Review.
-
Effect of Lumacaftor/Ivacaftor on Pulmonary Exacerbation Rates in Members with Cystic Fibrosis in a Medicaid Population.J Manag Care Spec Pharm. 2019 Sep;25(9):1021-1025. doi: 10.18553/jmcp.2019.25.9.1021. J Manag Care Spec Pharm. 2019. PMID: 31456498 Free PMC article.
Cited by
-
Addressing a broken drug pipeline for preterm birth: why early preterm birth is an orphan disease.Am J Obstet Gynecol. 2023 Dec;229(6):647-655. doi: 10.1016/j.ajog.2023.07.042. Epub 2023 Jul 27. Am J Obstet Gynecol. 2023. PMID: 37516401 Free PMC article.
-
Are We Capturing the Socioeconomic Burden of Rare Genetic Disease? A Scoping Review of Economic Evaluations and Cost-of-Illness Studies.Pharmacoeconomics. 2023 Dec;41(12):1563-1588. doi: 10.1007/s40273-023-01308-0. Epub 2023 Aug 18. Pharmacoeconomics. 2023. PMID: 37594668
-
Cost-Effectiveness Analysis Methods Used in Evaluations of Treatment for Cystic Fibrosis: A Scoping Review.Pharmacoeconomics. 2025 Jul;43(7):711-721. doi: 10.1007/s40273-025-01497-w. Epub 2025 Apr 29. Pharmacoeconomics. 2025. PMID: 40301297
-
Ivacaftor-tezacaftor-elexacaftor, tezacaftor-ivacaftor and lumacaftor-ivacaftor for treating cystic fibrosis: a systematic review and economic evaluation.Health Technol Assess. 2025 May;29(19):1-111. doi: 10.3310/CPLD8546. Health Technol Assess. 2025. PMID: 40418577 Free PMC article.
-
Gene therapy for cystic fibrosis: new tools for precision medicine.J Transl Med. 2021 Oct 30;19(1):452. doi: 10.1186/s12967-021-03099-4. J Transl Med. 2021. PMID: 34717671 Free PMC article. Review.
References
-
- National Heart, Lung, and Blood Institute. Facts about cystic fibrosis. November 1995. Available at: https://www.cdc.gov/scienceambassador/documents/cystic-fibrosis-fact-she.... Accessed July 24, 2018.
-
- Cystic Fibrosis Foundation. About cystic fibrosis. Available at: https://www.cff.org/What-is-CF/About-Cystic-Fibrosis/. Accessed July 24, 2018.
-
- British Columbia, Healthlink BC. How cystic fibrosis affects digestion and the pancreas. May 4, 2017. Available at: https://www.healthlinkbc.ca/healthtopics/ug1494. Accessed August 27, 2018.
-
- Mayo Clinic. Cystic fibrosis: diagnosis & treatment. October 13, 2016. Available at: http://www.mayoclinic.org/diseases-conditions/cystic-fibrosis/diagnosis-.... Accessed July 24, 2018.
-
- Cystic Fibrosis Foundation. Considerations for the use of lumacaftor and ivacaftor fixed dose combination oral tablets (Orkambi) for the management of persons with cystic fibrosis and two F508del CFTR mutations. August 28, 2015. Available at: https://www.cff.org/PDF-Archive/Orkambi-Whitepaper/. Accessed July 24, 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical