Contribution of dorsal root ganglion octamer transcription factor 1 to neuropathic pain after peripheral nerve injury
- PMID: 30247265
- PMCID: PMC6344274
- DOI: 10.1097/j.pain.0000000000001405
Contribution of dorsal root ganglion octamer transcription factor 1 to neuropathic pain after peripheral nerve injury
Abstract
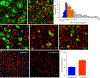
Neuropathic pain genesis is related to gene alterations in the dorsal root ganglion (DRG) after peripheral nerve injury. Transcription factors control gene expression. In this study, we investigated whether octamer transcription factor 1 (OCT1), a transcription factor, contributed to neuropathic pain caused by chronic constriction injury (CCI) of the sciatic nerve. Chronic constriction injury produced a time-dependent increase in the level of OCT1 protein in the ipsilateral L4/5 DRG, but not in the spinal cord. Blocking this increase through microinjection of OCT1 siRNA into the ipsilateral L4/5 DRG attenuated the initiation and maintenance of CCI-induced mechanical allodynia, heat hyperalgesia, and cold allodynia and improved morphine analgesia after CCI, without affecting basal responses to acute mechanical, heat, and cold stimuli as well as locomotor functions. Mimicking this increase through microinjection of recombinant adeno-associated virus 5 harboring full-length OCT1 into the unilateral L4/5 DRG led to marked mechanical allodynia, heat hyperalgesia, and cold allodynia in naive rats. Mechanistically, OCT1 participated in CCI-induced increases in Dnmt3a mRNA and its protein and DNMT3a-mediated decreases in Oprm1 and Kcna2 mRNAs and their proteins in the injured DRG. These findings indicate that OCT1 may participate in neuropathic pain at least in part by transcriptionally activating Dnmt3a and subsequently epigenetic silencing of Oprm1 and Kcan2 in the DRG. OCT1 may serve as a potential target for therapeutic treatments against neuropathic pain.
Conflict of interest statement
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures





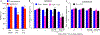
Similar articles
-
Dorsal root ganglion myeloid zinc finger protein 1 contributes to neuropathic pain after peripheral nerve trauma.Pain. 2015 Apr;156(4):711-721. doi: 10.1097/j.pain.0000000000000103. Pain. 2015. PMID: 25630025 Free PMC article.
-
Chronic constriction injury-induced microRNA-146a-5p alleviates neuropathic pain through suppression of IRAK1/TRAF6 signaling pathway.J Neuroinflammation. 2018 Jun 9;15(1):179. doi: 10.1186/s12974-018-1215-4. J Neuroinflammation. 2018. PMID: 29885668 Free PMC article.
-
FUS Contributes to Nerve Injury-Induced Nociceptive Hypersensitivity by Activating NF-κB Pathway in Primary Sensory Neurons.J Neurosci. 2023 Feb 15;43(7):1267-1278. doi: 10.1523/JNEUROSCI.2082-22.2022. Epub 2023 Jan 10. J Neurosci. 2023. PMID: 36627209 Free PMC article.
-
Transcription factor EBF1 mitigates neuropathic pain by rescuing Kv1.2 expression in primary sensory neurons.Transl Res. 2024 Jan;263:15-27. doi: 10.1016/j.trsl.2023.08.002. Epub 2023 Aug 20. Transl Res. 2024. PMID: 37607607 Free PMC article.
-
TET1 Overexpression Mitigates Neuropathic Pain Through Rescuing the Expression of μ-Opioid Receptor and Kv1.2 in the Primary Sensory Neurons.Neurotherapeutics. 2019 Apr;16(2):491-504. doi: 10.1007/s13311-018-00689-x. Neurotherapeutics. 2019. PMID: 30515739 Free PMC article.
Cited by
-
Communicating pain: emerging axonal signaling in peripheral neuropathic pain.Front Neuroanat. 2024 Jul 9;18:1398400. doi: 10.3389/fnana.2024.1398400. eCollection 2024. Front Neuroanat. 2024. PMID: 39045347 Free PMC article. Review.
-
[Role of ZHX2 in regulating dorsal root ganglion μ-opioid receptor expression in mice with peripheral nerve injuryinduced pain hypersensitivity].Nan Fang Yi Ke Da Xue Xue Bao. 2019 Aug 30;39(8):917-922. doi: 10.12122/j.issn.1673-4254.2019.08.07. Nan Fang Yi Ke Da Xue Xue Bao. 2019. PMID: 31511211 Free PMC article. Chinese.
-
N6-Methyladenosine Demethylase FTO Contributes to Neuropathic Pain by Stabilizing G9a Expression in Primary Sensory Neurons.Adv Sci (Weinh). 2020 May 27;7(13):1902402. doi: 10.1002/advs.201902402. eCollection 2020 Jul. Adv Sci (Weinh). 2020. PMID: 32670741 Free PMC article.
-
Intrathecal administration of the fat-mass and obesity-associated protein inhibitor mitigates neuropathic pain in female rats.Transl Perioper Pain Med. 2022;9(4):478-487. doi: 10.31480/2330-4871/163. Epub 2022 Nov 30. Transl Perioper Pain Med. 2022. PMID: 36545239 Free PMC article.
-
Nerve trauma-caused downregulation of opioid receptors in primary afferent neurons: Molecular mechanisms and potential managements.Exp Neurol. 2021 Mar;337:113572. doi: 10.1016/j.expneurol.2020.113572. Epub 2020 Dec 16. Exp Neurol. 2021. PMID: 33340498 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

