A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes
- PMID: 30247490
- PMCID: PMC6871539
- DOI: 10.1038/nbt.4245
A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes
Abstract
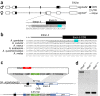
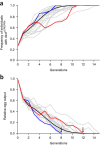
In the human malaria vector Anopheles gambiae, the gene doublesex (Agdsx) encodes two alternatively spliced transcripts, dsx-female (AgdsxF) and dsx-male (AgdsxM), that control differentiation of the two sexes. The female transcript, unlike the male, contains an exon (exon 5) whose sequence is highly conserved in all Anopheles mosquitoes so far analyzed. We found that CRISPR-Cas9-targeted disruption of the intron 4-exon 5 boundary aimed at blocking the formation of functional AgdsxF did not affect male development or fertility, whereas females homozygous for the disrupted allele showed an intersex phenotype and complete sterility. A CRISPR-Cas9 gene drive construct targeting this same sequence spread rapidly in caged mosquitoes, reaching 100% prevalence within 7-11 generations while progressively reducing egg production to the point of total population collapse. Owing to functional constraint of the target sequence, no selection of alleles resistant to the gene drive occurred in these laboratory experiments. Cas9-resistant variants arose in each generation at the target site but did not block the spread of the drive.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

