Human Breast-Milk Feeding Enhances the Humoral and Cell-Mediated Immune Response in Neonatal Piglets
- PMID: 30247686
- PMCID: PMC6209812
- DOI: 10.1093/jn/nxy170
Human Breast-Milk Feeding Enhances the Humoral and Cell-Mediated Immune Response in Neonatal Piglets
Abstract
Background: The benefits of breastfeeding infants are well characterized, including those on the immune system. However, determining the mechanism by which human breast milk (HBM) elicits effects on immune response requires investigation in an appropriate animal model.
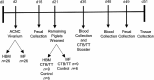
Objective: The primary aim of this study was to develop a novel porcine model and to determine the differential effects of feeding HBM and a commercial milk formula (MF) on immune response and gastrointestinal microbial colonization in a controlled environment.
Methods: Male piglets were fed HBM (n = 26) or MF (n = 26) from day 2 through day 21. Piglets were vaccinated (n = 9/diet group) with cholera toxin and cholera toxin subunit B (CTB) and tetanus toxoid at 21 d or were fed placebo (n = 6/diet group) and then weaned to a standard solid diet at the age of 21 d. Humoral and cell-mediated immune responses were assessed from blood on days 35 and 48. Immune response was further examined from tissues, including mesenteric lymph nodes (MLNs), Peyer's patches (PPs), and spleen. The colonization of gut microbiota was characterized from feces on days 16 and 49.
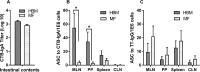
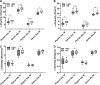
Results: Serum antibody titers in piglets fed HBM were 4-fold higher (P < 0.05) to CTB and 3-fold higher (P < 0.05) to tetanus toxoid compared with piglets fed MF on day 48. Compared with MF, the numbers of immunoglobulin A antibody-producing cells to CTB were 13-fold higher (P < 0.05) in MLNs and 11-fold higher (P < 0.05) in PPs in the HBM diet group on day 51. In addition, significantly increased T cell proliferation was observed in the HBM group relative to the MF group. Furthermore, microbial diversity in the HBM group was lower (P < 0.05) than in the MF group.
Conclusions: This porcine model appears to be valid for studying the effects of early postnatal diet on immune responses and the gastrointestinal microbiome. Our results lay the groundwork for future studies defining the role of infant diet on microbiota and immune function.
Figures
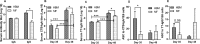


References
-
- Beaudry M, Dufour R, Marcoux S. Relation between infant feeding and infections during the first six months of life. J Pediatr 1995;126(2):191–7. - PubMed
-
- Beaudry M, Dufour R, Marcoux S. [Breast feeding and protection against infection in industrialized countries.] Arch Pediatr 1996;3(Suppl 1):126s–7s (in French). - PubMed
-
- Hanson LA, Korotkova M. The role of breastfeeding in prevention of neonatal infection. Semin Neonatol 2002;7(4):275–81. - PubMed
-
- Hanson LA, Korotkova M, Telemo E. Breast-feeding, infant formulas, and the immune system. Ann Allergy Asthma Immunol 2003;90(6 Suppl 3):59–63. - PubMed
-
- Hanson LA, Hofvander Y, Lindquist B, Zetterstrom R. Breast-feeding as a protection against gastroenteritis and other infections. Acta Paediatr Scand 1985;74(5):641–2. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

