NLRP6 negatively regulates pulmonary host defense in Gram-positive bacterial infection through modulating neutrophil recruitment and function
- PMID: 30248149
- PMCID: PMC6171945
- DOI: 10.1371/journal.ppat.1007308
NLRP6 negatively regulates pulmonary host defense in Gram-positive bacterial infection through modulating neutrophil recruitment and function
Abstract
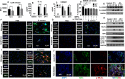
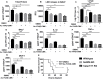
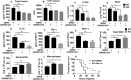
Gram-positive bacteria, including Staphylococcus aureus are endemic in the U.S., which cause life-threatening necrotizing pneumonia. Neutrophils are known to be critical for clearance of S. aureus infection from the lungs and extrapulmonary organs. Therefore, we investigated whether the NLRP6 inflammasome regulates neutrophil-dependent host immunity during pulmonary S. aureus infection. Unlike their wild-type (WT) counterparts, NLRP6 knockout (KO) mice were protected against pulmonary S. aureus infection as evidenced by their higher survival rate and lower bacterial burden in the lungs and extrapulmonary organs. In addition, NLRP6 KO mice displayed increased neutrophil recruitment following infection, and when neutrophils were depleted the protective effect was lost. Furthermore, neutrophils from the KO mice demonstrated enhanced intracellular bacterial killing and increased NADPH oxidase-dependent ROS production. Intriguingly, we found higher NK cell-mediated IFN-γ production in KO mouse lungs, and treatment with IFN-γ was found to enhance the bactericidal ability of WT and KO neutrophils. The NLRP6 KO mice also displayed decreased pyroptosis and necroptosis in the lungs following infection. Blocking of pyroptosis and necroptosis in WT mice resulted in increased survival, reduced bacterial burden in the lungs, and attenuated cytokine production. Taken together, these novel findings show that NLRP6 serves as a negative regulator of neutrophil-mediated host defense during Gram-positive bacterial infection in the lungs through regulating both neutrophil influx and function. These results also suggest that blocking NLRP6 to augment neutrophil-associated bacterial clearance should be considered as a potential therapeutic intervention strategy for treatment of S. aureus pneumonia.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- World Health Organization. Pneumonia fact sheet [Updated 2016 Sep; accessed 2017 Dec]. Available from http://www.who.int/mediacentre/factsheets/fs331/en/.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

