C-Tb skin test to diagnose Mycobacterium tuberculosis infection in children and HIV-infected adults: A phase 3 trial
- PMID: 30248152
- PMCID: PMC6152999
- DOI: 10.1371/journal.pone.0204554
C-Tb skin test to diagnose Mycobacterium tuberculosis infection in children and HIV-infected adults: A phase 3 trial
Abstract
Background: C-Tb, an ESAT-6/CFP-10-based skin test, has similar sensitivity for active TB compared to tuberculin skin test (TST) and QuantiFERON-TB-Gold-In-Tube (QFT). However, data are limited in children and HIV-infected persons.
Methods: Asymptomatic South African contacts <5 years (n = 87; HIV-uninfected), or symptomatic individuals of all ages presenting to clinics with suspected TB (n = 1003; 30% HIV-infected) were recruited from eight South African centres. C-Tb and TST were allocated to either forearm double blinded. Samples for QFT were collected in parallel, and test-positivity rates were compared.
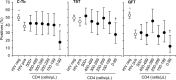
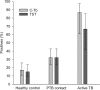
Results: In participants with microbiologically confirmed TB (n = 75; 45% HIV-infected) sensitivity of C-Tb, TST and QFT were similar (72% versus 75% versus 73%; p>0.5). All 3 tests had similar positivity rates in HIV-infected participants with active TB, however, positivity rates were reduced when CD4 counts were <100 cells/μL. In participants where active TB was excluded (n = 920), C-Tb (41%), TST (43%), and QFT (44%) also had similar test-positivity rates. Among asymptomatic contacts aged below five, 32% (28/87) tested positive with C-Tb and 32% (28/87) with TST (concordance 89%). Overall, C-Tb and TST showed a similar safety profile.
Conclusion: C-Tb was safe and showed similar test-positivity rates, compared to TST and QFT, in children and HIV-infected persons with active or latent M. tuberculosis infection. These data inform the utility of C-Tb in clinical practice.
Trial registration: ClinicalTrials.gov NCT01642888. EudraCT 2011-005078-40.
Conflict of interest statement
HA, MR, BBJ and PA are employed at SSI, a governmental non-profit research organization, who holds pending intellectual property rights over C-Tb. All intellectual property rights have been transferred to SSI. The other authors have declared no conflicts of interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- World Health Organization. Guidelines on the management of latent tuberculosis infection. 2015. Report No.: WHO/HTM/TB/2015.01. - PubMed
-
- Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol 1974. February;99(2):131–8. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

