Neurotensin and dynorphin Bi-Directionally modulate CeA inhibition of oval BNST neurons in male mice
- PMID: 30248304
- PMCID: PMC6438622
- DOI: 10.1016/j.neuropharm.2018.09.031
Neurotensin and dynorphin Bi-Directionally modulate CeA inhibition of oval BNST neurons in male mice
Abstract
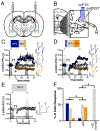
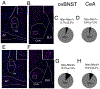
Neuropeptides are often co-expressed in neurons, and may therefore be working together to coordinate proper neural circuit function. However, neurophysiological effects of neuropeptides are commonly studied individually possibly underestimating their modulatory roles. Here, we triggered the release of endogenous neuropeptides in brain slices from male mice to better understand their modulation of central amygdala (CeA) inhibitory inputs onto oval (ov) BNST neurons. We found that locally-released neurotensin (NT) and dynorphin (Dyn) antagonistically regulated CeA inhibitory inputs onto ovBNST neurons. NT and Dyn respectively increased and decreased CeA-toovBNST inhibitory inputs through NT receptor 1 (NTR1) and kappa opioid receptor (KOR). Additionally, NT and Dyn mRNAs were highly co-localized in ovBNST neurons suggesting that they may be released from the same cells. Together, we showed that NT and Dyn are key modulators of CeA inputs to ovBNST, paving the way to determine whether different conditions or states can alter the neuropeptidergic regulation of this particular brain circuit.
Keywords: CeA; Dynorphin; GABA; Neuropeptides; Neurotensin; ovBNST.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Financial interest or conflict of interest: the authors have no financial or conflict of interest.
Figures




Similar articles
-
Dissecting the Roles of GABA and Neuropeptides from Rat Central Amygdala CRF Neurons in Anxiety and Fear Learning.Cell Rep. 2019 Oct 1;29(1):13-21.e4. doi: 10.1016/j.celrep.2019.08.083. Cell Rep. 2019. PMID: 31577943 Free PMC article.
-
A Key Role for Neurotensin in Chronic-Stress-Induced Anxiety-Like Behavior in Rats.Neuropsychopharmacology. 2018 Jan;43(2):285-293. doi: 10.1038/npp.2017.134. Epub 2017 Jun 26. Neuropsychopharmacology. 2018. PMID: 28649992 Free PMC article.
-
Presynaptic inhibition of gamma-aminobutyric acid release in the bed nucleus of the stria terminalis by kappa opioid receptor signaling.Biol Psychiatry. 2012 Apr 15;71(8):725-32. doi: 10.1016/j.biopsych.2011.11.015. Epub 2012 Jan 5. Biol Psychiatry. 2012. PMID: 22225848 Free PMC article.
-
Receptor-receptor interactions as studied with microdialysis. Focus on NTR/D2 interactions in the basal ganglia.J Neural Transm (Vienna). 2007 Jan;114(1):105-13. doi: 10.1007/s00702-006-0558-7. Epub 2006 Sep 19. J Neural Transm (Vienna). 2007. PMID: 16983483 Review.
-
Dynorphin/Kappa-Opioid Receptor System Modulation of Cortical Circuitry.Handb Exp Pharmacol. 2022;271:223-253. doi: 10.1007/164_2021_440. Handb Exp Pharmacol. 2022. PMID: 33580392 Review.
Cited by
-
Dynorphin/kappa opioid receptor system regulation on amygdaloid circuitry: Implications for neuropsychiatric disorders.Front Syst Neurosci. 2022 Oct 5;16:963691. doi: 10.3389/fnsys.2022.963691. eCollection 2022. Front Syst Neurosci. 2022. PMID: 36276608 Free PMC article. Review.
-
A novel GPR55-mediated satiety signal in the oval Bed Nucleus of the Stria Terminalis.Neuropsychopharmacology. 2019 Jun;44(7):1274-1283. doi: 10.1038/s41386-018-0309-0. Epub 2019 Jan 7. Neuropsychopharmacology. 2019. PMID: 30647449 Free PMC article.
-
Dynorphin and its role in alcohol use disorder.Brain Res. 2020 May 15;1735:146742. doi: 10.1016/j.brainres.2020.146742. Epub 2020 Feb 28. Brain Res. 2020. PMID: 32114059 Free PMC article. Review.
-
Dissecting the Roles of GABA and Neuropeptides from Rat Central Amygdala CRF Neurons in Anxiety and Fear Learning.Cell Rep. 2019 Oct 1;29(1):13-21.e4. doi: 10.1016/j.celrep.2019.08.083. Cell Rep. 2019. PMID: 31577943 Free PMC article.
-
Neurotensin in reward processes.Neuropharmacology. 2020 May 1;167:108005. doi: 10.1016/j.neuropharm.2020.108005. Epub 2020 Feb 11. Neuropharmacology. 2020. PMID: 32057800 Free PMC article. Review.
References
-
- Boudin H, Pelaprat D, Rostene W, Beaudet A, 1996. Cellular distribution of neurotensin receptors in rat brain: immunohistochemical study using an antipeptide antibody against the cloned high affinity receptor. J Comp Neurol 373, 76–89. - PubMed
-
- Cooke JH, Patterson M, Patel SR, Smith KL, Ghatei MA, Bloom SR, Murphy KG, 2009. Peripheral and central administration of xenin and neurotensin suppress food intake in rodents. Obesity (Silver Spring) 17, 1135–1143. - PubMed
-
- Dabrowska J, Hazra R, Guo JD, Li C, Dewitt S, Xu J, Lombroso PJ, Rainnie DG, 2013. Striatal-enriched protein tyrosine phosphatase-STEPs toward understanding chronic stress-induced activation of corticotrophin releasing factor neurons in the rat bed nucleus of the stria terminalis. Biol Psychiatry 74, 817–826. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

