Data-driven modeling of mitochondrial dysfunction in Alzheimer's disease
- PMID: 30248575
- PMCID: PMC6289702
- DOI: 10.1016/j.ceca.2018.09.003
Data-driven modeling of mitochondrial dysfunction in Alzheimer's disease
Abstract
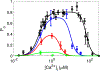
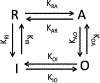
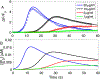
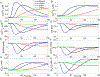
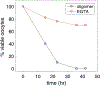
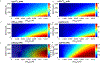
Intracellular accumulation of oligomeric forms of β amyloid (Aβ) are now believed to play a key role in the earliest phase of Alzheimer's disease (AD) as their rise correlates well with the early symptoms of the disease. Extensive evidence points to impaired neuronal Ca2+ homeostasis as a direct consequence of the intracellular Aβ oligomers. However, little is known about the downstream effects of the resulting Ca2+ rise on the many intracellular Ca2+-dependent pathways. Here we use multiscale modeling in conjunction with patch-clamp electrophysiology of single inositol 1,4,5-trisphosphate (IP3) receptor (IP3R) and fluorescence imaging of whole-cell Ca2+ response, induced by exogenously applied intracellular Aβ42 oligomers to show that Aβ42 inflicts cytotoxicity by impairing mitochondrial function. Driven by patch-clamp experiments, we first model the kinetics of IP3R, which is then extended to build a model for the whole-cell Ca2+ signals. The whole-cell model is then fitted to fluorescence signals to quantify the overall Ca2+ release from the endoplasmic reticulum by intracellular Aβ42 oligomers through G-protein-mediated stimulation of IP3 production. The estimated IP3 concentration as a function of intracellular Aβ42 content together with the whole-cell model allows us to show that Aβ42 oligomers impair mitochondrial function through pathological Ca2+ uptake and the resulting reduced mitochondrial inner membrane potential, leading to an overall lower ATP and increased production of reactive oxygen species and H2O2. We further show that mitochondrial function can be restored by the addition of Ca2+ buffer EGTA, in accordance with the observed abrogation of Aβ42 cytotoxicity by EGTA in our live cells experiments.
Keywords: Alzheimer's disease; Ca(2+) dyshomeostasis; Intracellular β amyloid; Mitochondrial dysfunction.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest
Authors declare no conflict of interest.
Figures

Similar articles
-
Aβ and NMDAR activation cause mitochondrial dysfunction involving ER calcium release.Neurobiol Aging. 2015 Feb;36(2):680-92. doi: 10.1016/j.neurobiolaging.2014.09.006. Epub 2014 Sep 6. Neurobiol Aging. 2015. PMID: 25442114
-
Nobiletin regulates intracellular Ca2+ levels via IP3R and ameliorates neuroinflammation in Aβ42-induced astrocytes.Redox Biol. 2024 Jul;73:103197. doi: 10.1016/j.redox.2024.103197. Epub 2024 May 16. Redox Biol. 2024. PMID: 38781730 Free PMC article.
-
Aβ42 oligomers selectively disrupt neuronal calcium release.Neurobiol Aging. 2015 Feb;36(2):877-85. doi: 10.1016/j.neurobiolaging.2014.10.020. Epub 2014 Oct 20. Neurobiol Aging. 2015. PMID: 25453559
-
Ca2+ homeostasis dysregulation in Alzheimer's disease: a focus on plasma membrane and cell organelles.FASEB J. 2019 Jun;33(6):6697-6712. doi: 10.1096/fj.201801751R. Epub 2019 Mar 8. FASEB J. 2019. PMID: 30848934 Review.
-
β-Amyloid and the Pathomechanisms of Alzheimer's Disease: A Comprehensive View.Molecules. 2017 Oct 10;22(10):1692. doi: 10.3390/molecules22101692. Molecules. 2017. PMID: 28994715 Free PMC article. Review.
Cited by
-
Quantifying the dose-dependent impact of intracellular amyloid beta in a mathematical model of calcium regulation in xenopus oocyte.PLoS One. 2021 Jan 28;16(1):e0246116. doi: 10.1371/journal.pone.0246116. eCollection 2021. PLoS One. 2021. PMID: 33508037 Free PMC article.
-
The Function of Mitochondrial Calcium Uniporter at the Whole-Cell and Single Mitochondrion Levels in WT, MICU1 KO, and MICU2 KO Cells.Cells. 2020 Jun 22;9(6):1520. doi: 10.3390/cells9061520. Cells. 2020. PMID: 32580385 Free PMC article.
-
Intracellular Injection of Brain Extracts from Alzheimer's Disease Patients Triggers Unregulated Ca2+ Release from Intracellular Stores That Hinders Cellular Bioenergetics.Cells. 2022 Nov 16;11(22):3630. doi: 10.3390/cells11223630. Cells. 2022. PMID: 36429057 Free PMC article.
-
The ubiquitous role of mitochondria in Parkinson and other neurodegenerative diseases.AIMS Neurosci. 2020 Mar 25;7(1):43-65. doi: 10.3934/Neuroscience.2020004. eCollection 2020. AIMS Neurosci. 2020. PMID: 32455165 Free PMC article. Review.
-
MAMs and Mitochondrial Quality Control: Overview and Their Role in Alzheimer's Disease.Neurochem Res. 2024 Oct;49(10):2682-2698. doi: 10.1007/s11064-024-04205-w. Epub 2024 Jul 13. Neurochem Res. 2024. PMID: 39002091 Review.
References
-
- Hardy JA, Higgins GA, Alzheimer’s disease: the amyloid cascade hypothesis, Science 256 (1992) 184. - PubMed
-
- Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB, et al., Amyloid β-peptide is produced by cultured cells during normal metabolism, Nature 359 (1992) 322–325. - PubMed
-
- Khachaturian Z, Calcium hypothesis of Alzheimer0s disease and brain aging, Ann. N. Y. Acad. Sci 747 (1994) 1–11. - PubMed
-
- Alzheimer’s ACHW, Calcium hypothesis of Alzheimer′s disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis., Alzheimers Dement. 13 (2017) 178. - PubMed
-
- Green KN, LaFerla FM, Linking calcium to Aβ and Alzheimer′s disease, Neuron 59 (2008) 190–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

