Vimentin Diversity in Health and Disease
- PMID: 30248895
- PMCID: PMC6210396
- DOI: 10.3390/cells7100147
Vimentin Diversity in Health and Disease
Abstract
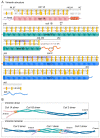
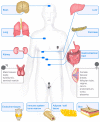
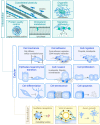
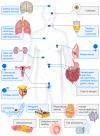
Vimentin is a protein that has been linked to a large variety of pathophysiological conditions, including cataracts, Crohn's disease, rheumatoid arthritis, HIV and cancer. Vimentin has also been shown to regulate a wide spectrum of basic cellular functions. In cells, vimentin assembles into a network of filaments that spans the cytoplasm. It can also be found in smaller, non-filamentous forms that can localise both within cells and within the extracellular microenvironment. The vimentin structure can be altered by subunit exchange, cleavage into different sizes, re-annealing, post-translational modifications and interacting proteins. Together with the observation that different domains of vimentin might have evolved under different selection pressures that defined distinct biological functions for different parts of the protein, the many diverse variants of vimentin might be the cause of its functional diversity. A number of review articles have focussed on the biology and medical aspects of intermediate filament proteins without particular commitment to vimentin, and other reviews have focussed on intermediate filaments in an in vitro context. In contrast, the present review focusses almost exclusively on vimentin, and covers both ex vivo and in vivo data from tissue culture and from living organisms, including a summary of the many phenotypes of vimentin knockout animals. Our aim is to provide a comprehensive overview of the current understanding of the many diverse aspects of vimentin, from biochemical, mechanical, cellular, systems biology and medical perspectives.
Keywords: biomechanics; cancer and metastasis; cell mechanical stiffness or elasticity; cellular contractility; cell‒extracellular matrix adhesions; clinical biomarkers; drug target; epithelial‒mesenchymal transition; extracellular vimentin; intermediate filaments; tissue regeneration; vimentin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

