NACC1, as a Target of MicroRNA-331-3p, Regulates Cell Proliferation in Urothelial Carcinoma Cells
- PMID: 30248959
- PMCID: PMC6210667
- DOI: 10.3390/cancers10100347
NACC1, as a Target of MicroRNA-331-3p, Regulates Cell Proliferation in Urothelial Carcinoma Cells
Abstract
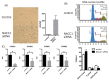
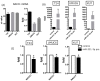
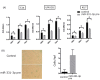
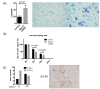
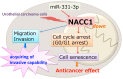
The nucleus accumbens-associated protein 1 (NACC1) is a transcription factor constitutively expressed in the urothelium, where it regulates cell growth, senescence, autophagy, and epithelial-mesenchymal transition. microRNA (miRNA) constitutes a class of small non-coding RNAs which are involved in cell proliferation, differentiation, and progression of tumors. miRNAs and their target molecules are utilized for molecular diagnosis of urothelial carcinoma. NACC1 is one of several putative target molecules of miR-331-3p, and is associated with cell proliferation in cancers such as prostate and cervical cancer. Functional experiments involving miR-331-3p and its target molecule NACC1 were conducted using the urothelial carcinoma (UC) cell lines, T24, UMUC6, and KU7. Furthermore, quantitative reverse transcription polymerase chain reaction and immunostaining were performed to evaluate the expression of NACC1 in UC derived from transurethral resection of bladder tumor (TUR-Bt) specimens. The methane thiosulfonate (MTS) assay revealed that cell proliferation was significantly reduced after transient transfection of miR-331-3p precursor and/or NACC1 siRNA in UC cells. Cell senescence via cell cycle arrest at the G1 phase was induced by NACC1 inhibition. On the other hand, suppression of NACC1 induced cell migration and invasion abilities. Immunohistochemical analysis of TUR-Bt specimens revealed that over 70% of UC cells presented strongly positive results for NACC1. In contrast, normal urothelial cells were weakly positive for NACC1. It was also found that NACC1 expression was lower in invasive UC cells than in non-invasive UC cells. Loss of NACC1 induced vessel invasion in invasive UC tissues. The present results indicate that NACC1 regulated by miR-331-3p contributes to cell proliferation, and is involved in cell migration and invasion. This suggests that NACC1 can serve as a potential target molecule for the prediction and prognosis of UC, and can contribute to effective treatment strategies.
Keywords: NACC1; cell cycle arrest; miR-331-3p; migration and invasion; urothelial carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
miR-218-5p, miR-124-3p and miR-23b-3p act synergistically to modulate the expression of NACC1, proliferation, and apoptosis in C-33A and CaSki cells.Noncoding RNA Res. 2024 Feb 27;9(3):720-731. doi: 10.1016/j.ncrna.2024.02.016. eCollection 2024 Sep. Noncoding RNA Res. 2024. PMID: 38577025 Free PMC article.
-
Overexpression of MicroRNA-138 Affects the Proliferation and Invasion of Urothelial Carcinoma Cells by Suppressing SOX9 Expression.Biomedicines. 2023 Nov 15;11(11):3064. doi: 10.3390/biomedicines11113064. Biomedicines. 2023. PMID: 38002064 Free PMC article.
-
Downregulation of N-Acetylglucosaminyltransferase GCNT3 by miR-302b-3p Decreases Non-Small Cell Lung Cancer (NSCLC) Cell Proliferation, Migration and Invasion.Cell Physiol Biochem. 2018;50(3):987-1004. doi: 10.1159/000494482. Epub 2018 Oct 24. Cell Physiol Biochem. 2018. PMID: 30355927
-
An intellectual-disability-associated mutation of the transcriptional regulator NACC1 impairs glutamatergic neurotransmission.Front Mol Neurosci. 2023 Jul 14;16:1115880. doi: 10.3389/fnmol.2023.1115880. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37533751 Free PMC article. Review.
-
Epithelial plasticity in urothelial carcinoma: Current advancements and future challenges.World J Stem Cells. 2016 Aug 26;8(8):260-7. doi: 10.4252/wjsc.v8.i8.260. World J Stem Cells. 2016. PMID: 27621760 Free PMC article. Review.
Cited by
-
MicroRNA-331 inhibits development of gastric cancer through targeting musashi1.World J Gastrointest Oncol. 2019 Sep 15;11(9):705-716. doi: 10.4251/wjgo.v11.i9.705. World J Gastrointest Oncol. 2019. PMID: 31558975 Free PMC article.
-
miR-218-5p, miR-124-3p and miR-23b-3p act synergistically to modulate the expression of NACC1, proliferation, and apoptosis in C-33A and CaSki cells.Noncoding RNA Res. 2024 Feb 27;9(3):720-731. doi: 10.1016/j.ncrna.2024.02.016. eCollection 2024 Sep. Noncoding RNA Res. 2024. PMID: 38577025 Free PMC article.
-
miR-325-3p Overexpression Inhibits Proliferation and Metastasis of Bladder Cancer Cells by Regulating MT3.Med Sci Monit. 2020 Jun 8;26:e920331. doi: 10.12659/MSM.920331. Med Sci Monit. 2020. PMID: 32512576 Free PMC article.
-
The Emerging Roles of Autophagy-Related MicroRNAs in Cancer.Int J Biol Sci. 2021 Jan 1;17(1):134-150. doi: 10.7150/ijbs.50773. eCollection 2021. Int J Biol Sci. 2021. PMID: 33390839 Free PMC article. Review.
-
Tumorous expression of NAC1 restrains antitumor immunity through the LDHA-mediated immune evasion.J Immunother Cancer. 2022 Sep;10(9):e004856. doi: 10.1136/jitc-2022-004856. J Immunother Cancer. 2022. PMID: 36150745 Free PMC article.
References
-
- Ro J.Y., Staerkel G.A., Ayala A.G. Cytologic and histologic features of superficial bladder cancer. Urol. Clin. N. Am. 1992;19:435–453. - PubMed
-
- Rahman M.T., Nakayama K., Rahman M., Katagiri H., Katagiri A., Ishibashi T., Ishikawa M., Iida K., Nakayama N., Otsuki Y., et al. Fatty acid synthase expression associated with nac1 is a potential therapeutic target in ovarian clear cell carcinomas. Br. J. Cancer. 2012;107:300–307. doi: 10.1038/bjc.2012.246. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

