Effect of levothyroxine supplementation on pregnancy outcomes in women with subclinical hypothyroidism and thyroid autoimmuneity undergoing in vitro fertilization/intracytoplasmic sperm injection: an updated meta-analysis of randomized controlled trials
- PMID: 30249251
- PMCID: PMC6154908
- DOI: 10.1186/s12958-018-0410-6
Effect of levothyroxine supplementation on pregnancy outcomes in women with subclinical hypothyroidism and thyroid autoimmuneity undergoing in vitro fertilization/intracytoplasmic sperm injection: an updated meta-analysis of randomized controlled trials
Abstract
Background: Evidence suggests that subclinical hypothyroidism (SCH) and thyroid autoimmunity (TAI) are associated with adverse pregnancy outcomes. This systematic review and meta-analysis was conducted to determine whether levothyroxine (LT4) supplementation would improve pregnancy outcomes among infertile women with SCH and/or TAI who underwent in vitro fertilization (IVF) or intracytoplastic sperm injection (ICSI).
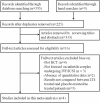
Methods: We searched databases of PubMed, EMBASE, Web of Science, Cochrane Controlled Trials Register databases, and Clinicaltrials.gov up to April 2018 to identify eligible studies. Studies that focused on the treatment effect of LT4 on pregnancy outcomes of women with SCH and/or TAI who underwent IVF/ICSI were included in the data synthesis. We only included randomized controlled trials (RCTs). Relative risks (RR) and 95% confidence intervals (CI) were calculated using a random-effects model to assess the results of pregnancy outcomes, including clinical pregnancy rate, miscarriage rate, live birth rate and preterm birth rate.
Results: Four published RCTs including 787 infertile couples undergoing IVF/ICSI were included in this meta-analysis. Notably, the study observed no significant associations of LT4 treatment with the clinical pregnancy rate (RR = 1.46, 95% CI: 0.86-2.48), live birth rate (RR = 2.05, 95% CI: 0.96-4.36), or preterm birth rate (RR = 1.13, 95% CI: 0.65-1.96). However, patients receiving LT4 supplementation had a significantly decreased miscarriage rate relative to those receiving a placebo or no treatment (RR = 0.51, 95% CI: 0.32-0.82). A further sub-group analysis showed that LT4 supplementation did not improve the miscarriage rates among patients with SCH (RR = 0.67, 95% CI: 0.39-1.15) or TAI (RR = 0.28, 95% CI: 0.07-1.06).
Conclusions: Given its potential to reduce the miscarriage rate, LT4 supplementation is recommended for infertile women with SCH and/or TAI who are undergoing IVF/ICSI. However, additional population-based RCTs are needed to confirm this recommendation.
Keywords: IVF/ICSI; Levothyroxine; Pregnancy outcome; Subclinical hypothyroidism; Thyroid autoimmunity.
Conflict of interest statement
Competing interest
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI).Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD012693. doi: 10.1002/14651858.CD012693.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2024 Jan 4;1:CD012693. doi: 10.1002/14651858.CD012693.pub3. PMID: 29388198 Free PMC article. Updated.
-
Application of seminal plasma to female genital tract prior to embryo transfer in assisted reproductive technology cycles (IVF, ICSI and frozen embryo transfer).Cochrane Database Syst Rev. 2018 Feb 28;2(2):CD011809. doi: 10.1002/14651858.CD011809.pub2. Cochrane Database Syst Rev. 2018. PMID: 29489026 Free PMC article.
-
Antioxidants for male subfertility.Cochrane Database Syst Rev. 2022 May 4;5(5):CD007411. doi: 10.1002/14651858.CD007411.pub5. Cochrane Database Syst Rev. 2022. PMID: 35506389 Free PMC article.
-
Effects of Levothyroxine Treatment on Fertility and Pregnancy Outcomes in Subclinical Hypothyroidism: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Thyroid. 2024 Apr;34(4):519-530. doi: 10.1089/thy.2023.0546. Thyroid. 2024. PMID: 38368537
-
Effect of levothyroxine supplementation on pregnancy loss and preterm birth in women with subclinical hypothyroidism and thyroid autoimmunity: a systematic review and meta-analysis.Hum Reprod Update. 2019 May 1;25(3):344-361. doi: 10.1093/humupd/dmz003. Hum Reprod Update. 2019. PMID: 30951172
Cited by
-
Thyroid dysfunction in Iranian pregnant women: a systematic review and meta-analysis.BMC Pregnancy Childbirth. 2020 Jul 14;20(1):405. doi: 10.1186/s12884-020-03040-5. BMC Pregnancy Childbirth. 2020. PMID: 32664874 Free PMC article.
-
TSH levels after fresh embryo transfer are associated with reproductive outcomes in euthyroid women undergoing the first IVF/ICSI cycles.Sci Rep. 2023 Jun 2;13(1):8963. doi: 10.1038/s41598-023-36276-0. Sci Rep. 2023. PMID: 37268813 Free PMC article.
-
Serum and follicular fluid thyroid hormone levels and assisted reproductive technology outcomes.Reprod Biol Endocrinol. 2019 Nov 7;17(1):90. doi: 10.1186/s12958-019-0529-0. Reprod Biol Endocrinol. 2019. PMID: 31699106 Free PMC article.
-
Reproductive Outcomes in Cases of Subclinical Hypothyroidism and Thyroid Autoimmunity: A Narrative Review.Rev Bras Ginecol Obstet. 2020 Dec;42(12):829-833. doi: 10.1055/s-0040-1714133. Epub 2020 Dec 21. Rev Bras Ginecol Obstet. 2020. PMID: 33348400 Free PMC article. Review.
-
Effect of antithyroid antibodies on women with recurrent miscarriage: A meta-analysis.Am J Reprod Immunol. 2020 Jun;83(6):e13238. doi: 10.1111/aji.13238. Epub 2020 Apr 11. Am J Reprod Immunol. 2020. PMID: 32198952 Free PMC article. Review.
References
-
- Maraka Spyridoula, Ospina Naykky M. Singh, O'Keeffe Derek T., Espinosa De Ycaza Ana E., Gionfriddo Michael R., Erwin Patricia J., Coddington Charles C., Stan Marius N., Murad M. Hassan, Montori Victor M. Subclinical Hypothyroidism in Pregnancy: A Systematic Review and Meta-Analysis. Thyroid. 2016;26(4):580–590. doi: 10.1089/thy.2015.0418. - DOI - PMC - PubMed
-
- Haddow JE, Cleary-Goldman J, McClain MR, Palomaki GE, Neveux LM, Lambert-Messerlian G, Canick JA, Malone FD, Porter TF, Nyberg DA, Bernstein PS, D'Alton ME. First, second-trimester risk of aneuploidy research C. Thyroperoxidase and thyroglobulin antibodies in early pregnancy and preterm delivery. Obstet Gynecol. 2010;116:58–62. doi: 10.1097/AOG.0b013e3181e10b30. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous