Loss of males from mixed-sex societies in termites
- PMID: 30249269
- PMCID: PMC6154949
- DOI: 10.1186/s12915-018-0563-y
Loss of males from mixed-sex societies in termites
Abstract
Background: Sexual reproduction is the norm in almost all animal species, and in many advanced animal societies, both males and females participate in social activities. To date, the complete loss of males from advanced social animal lineages has been reported only in ants and honey bees (Hymenoptera), whose workers are always female and whose males display no helping behaviors even in normal sexual species. Asexuality has not previously been observed in colonies of another major group of social insects, the termites, where the ubiquitous presence of both male and female workers and soldiers indicate that males play a critical role beyond that of reproduction.
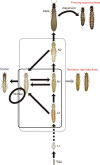
Results: Here, we report asexual societies in a lineage of the termite Glyptotermes nakajimai. We investigated the composition of mature colonies from ten distinct populations in Japan, finding six asexual populations characterized by a lack of any males in the reproductive, soldier, and worker castes of their colonies, an absence of sperm in the spermathecae of their queens, and the development of unfertilized eggs at a level comparable to that for the development of fertilized eggs in sexual populations of this species. Phylogenetic analyses indicated a single evolutionary origin of the asexual populations, with divergence from sampled sexual populations occurring about 14 million years ago. Asexual colonies differ from sexual colonies in having a more uniform head size in their all-female soldier caste, and fewer soldiers in proportion to other individuals, suggesting increased defensive efficiencies arising from uniform soldier morphology. Such efficiencies may have contributed to the persistence and spread of the asexual lineage. Cooperative colony foundation by multiple queens, the single-site nesting life history common to both the asexual and sexual lineages, and the occasional development of eggs without fertilization even in the sexual lineage are traits likely to have been present in the ancestors of the asexual lineage that may have facilitated the transition to asexuality.
Conclusions: Our findings demonstrate that completely asexual social lineages can evolve from mixed-sex termite societies, providing evidence that males are dispensable for the maintenance of advanced animal societies in which they previously played an active social role.
Keywords: Advanced social animals; All-female asexual societies; Asexual social lineages; Sexual reproduction; Social insects; Thelytokous parthenogenesis.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






Comment in
-
The disposable male- the ultimate emancipation of females?BMC Biol. 2018 Sep 25;16(1):106. doi: 10.1186/s12915-018-0574-8. BMC Biol. 2018. PMID: 30249271 Free PMC article.
Similar articles
-
Widespread occurrence of asexual reproduction in higher termites of the Termes group (Termitidae: Termitinae).BMC Evol Biol. 2019 Jun 21;19(1):131. doi: 10.1186/s12862-019-1459-3. BMC Evol Biol. 2019. PMID: 31226928 Free PMC article.
-
Enhanced heterozygosity from male meiotic chromosome chains is superseded by hybrid female asexuality in termites.Proc Natl Acad Sci U S A. 2021 Dec 21;118(51):e2009533118. doi: 10.1073/pnas.2009533118. Proc Natl Acad Sci U S A. 2021. PMID: 34903643 Free PMC article.
-
Comparative screening of endosymbiotic bacteria associated with the asexual and sexual lineages of the termite Glyptotermes nakajimai.Commun Integr Biol. 2019 Mar 20;12(1):55-58. doi: 10.1080/19420889.2019.1592418. eCollection 2019. Commun Integr Biol. 2019. PMID: 31143363 Free PMC article.
-
Evolution of the asexual queen succession system and its underlying mechanisms in termites.J Exp Biol. 2017 Jan 1;220(Pt 1):63-72. doi: 10.1242/jeb.142547. J Exp Biol. 2017. PMID: 28057829 Review.
-
Unusual modes of reproduction in social insects: shedding light on the evolutionary paradox of sex.Bioessays. 2011 Dec;33(12):927-37. doi: 10.1002/bies.201100096. Epub 2011 Oct 14. Bioessays. 2011. PMID: 21997278 Review.
Cited by
-
Widespread occurrence of asexual reproduction in higher termites of the Termes group (Termitidae: Termitinae).BMC Evol Biol. 2019 Jun 21;19(1):131. doi: 10.1186/s12862-019-1459-3. BMC Evol Biol. 2019. PMID: 31226928 Free PMC article.
-
Enhanced heterozygosity from male meiotic chromosome chains is superseded by hybrid female asexuality in termites.Proc Natl Acad Sci U S A. 2021 Dec 21;118(51):e2009533118. doi: 10.1073/pnas.2009533118. Proc Natl Acad Sci U S A. 2021. PMID: 34903643 Free PMC article.
-
Comparative screening of endosymbiotic bacteria associated with the asexual and sexual lineages of the termite Glyptotermes nakajimai.Commun Integr Biol. 2019 Mar 20;12(1):55-58. doi: 10.1080/19420889.2019.1592418. eCollection 2019. Commun Integr Biol. 2019. PMID: 31143363 Free PMC article.
-
Diversity of Termite Breeding Systems.Insects. 2019 Feb 12;10(2):52. doi: 10.3390/insects10020052. Insects. 2019. PMID: 30759735 Free PMC article. Review.
-
Caste development and sex ratio of the Ryukyu drywood termite Neotermes sugioi and its potential mechanisms.Sci Rep. 2021 Jul 22;11(1):15037. doi: 10.1038/s41598-021-94505-w. Sci Rep. 2021. PMID: 34294796 Free PMC article.
References
-
- Bell G. The masterpiece of nature: the evolution and genetics of sexuality. San Francisco: University of California Press; 1982.
-
- West SA, Lively CM, Read AF. A pluralist approach to sex and recombination. J Evol Biol. 1999;12:1003–1012. doi: 10.1046/j.1420-9101.1999.00119.x. - DOI
-
- Burt A. Perspective: sex, recombination, and the efficacy of selection—was Weismann right? Evolution. 2000;54:337–351. - PubMed
-
- Normark BB, Judson OP, Moran NA. Genomic signatures of ancient asexual lineages. Biol J Linn Soc. 2003;79:69–84. doi: 10.1046/j.1095-8312.2003.00182.x. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

