Small molecule inhibits α-synuclein aggregation, disrupts amyloid fibrils, and prevents degeneration of dopaminergic neurons
- PMID: 30249646
- PMCID: PMC6187188
- DOI: 10.1073/pnas.1804198115
Small molecule inhibits α-synuclein aggregation, disrupts amyloid fibrils, and prevents degeneration of dopaminergic neurons
Abstract
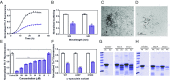
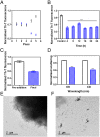
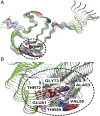
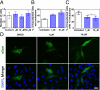
Parkinson's disease (PD) is characterized by a progressive loss of dopaminergic neurons, a process that current therapeutic approaches cannot prevent. In PD, the typical pathological hallmark is the accumulation of intracellular protein inclusions, known as Lewy bodies and Lewy neurites, which are mainly composed of α-synuclein. Here, we exploited a high-throughput screening methodology to identify a small molecule (SynuClean-D) able to inhibit α-synuclein aggregation. SynuClean-D significantly reduces the in vitro aggregation of wild-type α-synuclein and the familiar A30P and H50Q variants in a substoichiometric molar ratio. This compound prevents fibril propagation in protein-misfolding cyclic amplification assays and decreases the number of α-synuclein inclusions in human neuroglioma cells. Computational analysis suggests that SynuClean-D can bind to cavities in mature α-synuclein fibrils and, indeed, it displays a strong fibril disaggregation activity. The treatment with SynuClean-D of two PD Caenorhabditis elegans models, expressing α-synuclein either in muscle or in dopaminergic neurons, significantly reduces the toxicity exerted by α-synuclein. SynuClean-D-treated worms show decreased α-synuclein aggregation in muscle and a concomitant motility recovery. More importantly, this compound is able to rescue dopaminergic neurons from α-synuclein-induced degeneration. Overall, SynuClean-D appears to be a promising molecule for therapeutic intervention in Parkinson's disease.
Keywords: Parkinson’s disease; aggregation inhibition; dopaminergic degeneration; protein aggregation; α-synuclein.
Conflict of interest statement
Conflict of interest statement: J.P., S.P.-D., M.C.-G., J.S., E.D., and S.V. are inventors on a patent application (PCT/EP2018/054540) related to the compound in this study.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

