Comparative Analysis of the Capacity of the Candida Species To Elicit Vaginal Immunopathology
- PMID: 30249743
- PMCID: PMC6246903
- DOI: 10.1128/IAI.00527-18
Comparative Analysis of the Capacity of the Candida Species To Elicit Vaginal Immunopathology
Abstract
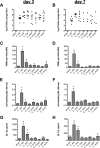
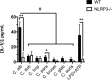
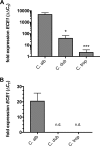
The human fungal pathogen Candida albicans is the major etiological agent of vulvovaginal candidiasis (VVC). Despite this fact, other non-albicans Candida (NAC) species have frequently been reported, as well. Despite their presence in the vaginal environment, little is known about their capacities to elicit immune responses classically associated with C. albicans-mediated immunopathology, including neutrophil recruitment and proinflammatory cytokine signaling. Therefore, using a combination of in vitro and in vivo approaches, we undertook a comparative analysis to determine whether a representative panel of NAC species could colonize, induce immunopathological markers, or cause damage at the vaginal mucosa. Using a murine model of VVC, C. albicans was found to induce robust immunopathology (neutrophils and interleukin 1β [IL-1β]) and elicit mucosal damage. However, all the NAC species tested (including C. dubliniensis, C. tropicalis, C. parapsilosis, C. krusei, C. glabrata, and C. auris) induced significantly less damage and neutrophil recruitment than C. albicans, despite achieving similar early colonization levels. These results largely correlated with a notable lack of ability by the NAC species (including C. dubliniensis and C. tropicalis) to form hyphae both in vitro and in vivo Furthermore, both C. dubliniensis and C. tropicalis induced significantly less expression of the ECE1 gene encoding candidalysin, a key fungal virulence determinant driving VVC immunopathology. In order to determine the relative capacities of these species to elicit inflammasome-dependent IL-1β release, both wild-type and NLRP3-/- THP-1 cells were challenged in vitro While most species tested elicited only modest amounts of IL-1β, challenge with C. albicans led to significantly elevated levels that were largely NLRP3 dependent. Collectively, our findings demonstrate that although NAC species are increasingly reported as causative agents of VVC, C. albicans appears to be exceedingly vaginopathogenic, exhibiting robust immunopathology, hypha formation, and candidalysin expression. Thus, this study provides mechanistic insight into why C. albicans is overwhelmingly the major pathogen reported during VVC.
Keywords: Candida; NAC species; VVC; immunopathogenesis; inflammasome; vaginitis; vulvovaginal.
Copyright © 2018 American Society for Microbiology.
Figures

Similar articles
-
Transcriptomic analysis of vulvovaginal candidiasis identifies a role for the NLRP3 inflammasome.mBio. 2015 Apr 21;6(2):e00182-15. doi: 10.1128/mBio.00182-15. mBio. 2015. PMID: 25900651 Free PMC article.
-
Candidalysin Drives Epithelial Signaling, Neutrophil Recruitment, and Immunopathology at the Vaginal Mucosa.Infect Immun. 2018 Jan 22;86(2):e00645-17. doi: 10.1128/IAI.00645-17. Print 2018 Feb. Infect Immun. 2018. PMID: 29109176 Free PMC article.
-
Candidalysin Crucially Contributes to Nlrp3 Inflammasome Activation by Candida albicans Hyphae.mBio. 2019 Jan 8;10(1):e02221-18. doi: 10.1128/mBio.02221-18. mBio. 2019. PMID: 30622184 Free PMC article.
-
Novel Mechanism behind the Immunopathogenesis of Vulvovaginal Candidiasis: "Neutrophil Anergy".Infect Immun. 2018 Feb 20;86(3):e00684-17. doi: 10.1128/IAI.00684-17. Print 2018 Mar. Infect Immun. 2018. PMID: 29203543 Free PMC article. Review.
-
Biofilms and vulvovaginal candidiasis.Colloids Surf B Biointerfaces. 2019 Feb 1;174:110-125. doi: 10.1016/j.colsurfb.2018.11.011. Epub 2018 Nov 7. Colloids Surf B Biointerfaces. 2019. PMID: 30447520 Review.
Cited by
-
α-galactosylceramide-stimulated invariant natural killer T-cells play a protective role in murine vulvovaginal candidiasis by Candida albicans.PLoS One. 2021 Nov 16;16(11):e0259306. doi: 10.1371/journal.pone.0259306. eCollection 2021. PLoS One. 2021. PMID: 34784362 Free PMC article.
-
It Takes Two to Tango: How a Dysregulation of the Innate Immunity, Coupled With Candida Virulence, Triggers VVC Onset.Front Microbiol. 2021 Jun 7;12:692491. doi: 10.3389/fmicb.2021.692491. eCollection 2021. Front Microbiol. 2021. PMID: 34163460 Free PMC article. Review.
-
Impaired neutrophil extracellular trap-forming capacity contributes to susceptibility to chronic vaginitis in a mouse model of vulvovaginal candidiasis.Infect Immun. 2024 Mar 12;92(3):e0035023. doi: 10.1128/iai.00350-23. Epub 2024 Jan 30. Infect Immun. 2024. PMID: 38289125 Free PMC article.
-
Vulvovaginal candidiasis culture results from a major national commercial laboratory, United States, 2019-2023.Am J Obstet Gynecol. 2024 Sep;231(3):e101-e104. doi: 10.1016/j.ajog.2024.04.048. Epub 2024 May 6. Am J Obstet Gynecol. 2024. PMID: 38710271 No abstract available.
-
Nanobody-mediated neutralization of candidalysin prevents epithelial damage and inflammatory responses that drive vulvovaginal candidiasis pathogenesis.mBio. 2024 Mar 13;15(3):e0340923. doi: 10.1128/mbio.03409-23. Epub 2024 Feb 13. mBio. 2024. PMID: 38349176 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

