Autoinducer 2 (AI-2) Production by Nontypeable Haemophilus influenzae 86-028NP Promotes Expression of a Predicted Glycosyltransferase That Is a Determinant of Biofilm Maturation, Prevention of Dispersal, and Persistence In Vivo
- PMID: 30249749
- PMCID: PMC6246909
- DOI: 10.1128/IAI.00506-18
Autoinducer 2 (AI-2) Production by Nontypeable Haemophilus influenzae 86-028NP Promotes Expression of a Predicted Glycosyltransferase That Is a Determinant of Biofilm Maturation, Prevention of Dispersal, and Persistence In Vivo
Abstract
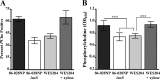
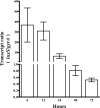
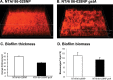
Nontypeable Haemophilus influenzae (NTHi) is an extremely common human pathobiont that persists on the airway mucosal surface within biofilm communities, and our previous work has shown that NTHi biofilm maturation is coordinated by the production and uptake of autoinducer 2 (AI-2) quorum signals. To directly test roles for AI-2 in maturation and maintenance of NTHi biofilms, we generated an NTHi 86-028NP mutant in which luxS transcription was under the control of the xylA promoter (NTHi 86-028NP luxS xylA::luxS), rendering AI-2 production inducible by xylose. Comparison of biofilms under inducing and noninducing conditions revealed a biofilm defect in the absence of xylose, whereas biofilm maturation increased following xylose induction. The removal of xylose resulted in the interruption of luxS expression and biofilm dispersal. Measurement of luxS transcript levels by real-time reverse transcription-PCR (RT-PCR) showed that luxS expression peaked as biofilms matured and waned before dispersal. Transcript profiling revealed significant changes following the induction of luxS, including increased transcript levels for a predicted family 8 glycosyltransferase (NTHI1750; designated gstA); this result was confirmed by real-time RT-PCR. An isogenic NTHi 86-028NP gstA mutant had a biofilm defect, including decreased levels of sialylated matrix and significantly altered biofilm structure. In experimental chinchilla infections, we observed a significant decrease in the number of bacteria in the biofilm population (but not in effusions) for NTHi 86-028NP gstA compared to the parental strain. Therefore, we conclude that AI-2 promotes NTHi biofilm maturation and the maintenance of biofilm integrity, due at least in part to the expression of a probable glycosyltransferase that is potentially involved in the synthesis of the biofilm matrix.
Keywords: Haemophilus influenzae; biofilm; otitis media; quorum signal.
Copyright © 2018 American Society for Microbiology.
Figures






References
-
- Mackenzie GA, Leach AJ, Carapetis JR, Fisher J, Morris PS. 2010. Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease. BMC Infect Dis 10:304. doi:10.1186/1471-2334-10-304. - DOI - PMC - PubMed
-
- Peltola H. 2000. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev 13:302–317. doi:10.1128/CMR.13.2.302. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

