Mechanics of cortical folding: stress, growth and stability
- PMID: 30249772
- PMCID: PMC6158197
- DOI: 10.1098/rstb.2017.0321
Mechanics of cortical folding: stress, growth and stability
Abstract
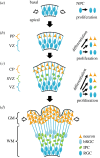
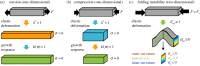
Cortical folding, or gyrification, coincides with several important developmental processes. The folded shape of the human brain allows the cerebral cortex, the thin outer layer of neurons and their associated projections, to attain a large surface area relative to brain volume. Abnormal cortical folding has been associated with severe neurological, cognitive and behavioural disorders, such as epilepsy, autism and schizophrenia. However, despite decades of study, the mechanical forces that lead to cortical folding remain incompletely understood. Leading hypotheses have focused on the roles of (i) tangential growth of the outer cortex, (ii) spatio-temporal patterns in the birth and migration of neurons, and (iii) internal tension in axons. Recent experimental studies have illuminated not only the fundamental cellular and molecular processes underlying cortical development, but also the stress state, mechanical properties and spatio-temporal patterns of growth in the developing brain. The combination of mathematical modelling and physical measurements has allowed researchers to evaluate hypothesized mechanisms of folding, to determine whether each is consistent with physical laws. This review summarizes what physical scientists have learned from models and recent experimental observations, in the context of recent neurobiological discoveries regarding cortical development. Here, we highlight evidence of a combined mechanism, in which spatio-temporal patterns bias the locations of primary folds (i), but tangential growth of the cortical plate induces mechanical instability (ii) to propagate primary and higher-order folds.This article is part of the Theo Murphy meeting issue 'Mechanics of development'.
Keywords: cortical folding; growth; gyrification; instability; modelling; stress.
© 2018 The Author(s).
Conflict of interest statement
We declare we have no competing interests.
Figures




References
-
- Welker W. 1990. Why does the cortex fissure and fold: a review of determinants of gyri and sulci. In Cerebral cortex: comparative structure and evolution of cerebral cortex (eds Jones EG, Peters A), pp. 3–136. New York, NY: Plenum Press.
-
- Le Gros Clark WE. 1945. Deformation patterns in the cerebral cortex. In Essays on growth and form (eds Le Gros Clark WE, Medawar PB), pp. 1–22. London, UK: Oxford University Press.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
