The Avian Basal Ganglia Are a Source of Rapid Behavioral Variation That Enables Vocal Motor Exploration
- PMID: 30249800
- PMCID: PMC6222063
- DOI: 10.1523/JNEUROSCI.2915-17.2018
The Avian Basal Ganglia Are a Source of Rapid Behavioral Variation That Enables Vocal Motor Exploration
Abstract
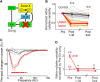
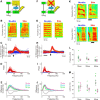
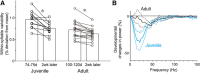
The basal ganglia (BG) participate in aspects of reinforcement learning that require evaluation and selection of motor programs associated with improved performance. However, whether the BG additionally contribute to behavioral variation ("motor exploration") that forms the substrate for such learning remains unclear. In songbirds, a tractable system for studying BG-dependent skill learning, a role for the BG in generating exploratory variability, has been challenged by the finding that lesions of Area X, the song-specific component of the BG, have no lasting effects on several forms of vocal variability that have been studied. Here we demonstrate that lesions of Area X in adult male zebra finches (Taeniopygia gutatta) permanently eliminate rapid within-syllable variation in fundamental frequency (FF), which can act as motor exploration to enable reinforcement-driven song learning. In addition, we found that this within-syllable variation is elevated in juveniles and in adults singing alone, conditions that have been linked to enhanced song plasticity and elevated neural variability in Area X. Consistent with a model that variability is relayed from Area X, via its cortical target, the lateral magnocellular nucleus of the anterior nidopallium (LMAN), to influence song motor circuitry, we found that lesions of LMAN also eliminate within-syllable variability. Moreover, we found that electrical perturbation of LMAN can drive fluctuations in FF that mimic naturally occurring within-syllable variability. Together, these results demonstrate that the BG are a central source of rapid behavioral variation that can serve as motor exploration for vocal learning.SIGNIFICANCE STATEMENT Many complex motor skills, such as speech, are not innately programmed but are learned gradually through trial and error. Learning involves generating exploratory variability in action ("motor exploration") and evaluating subsequent performance to acquire motor programs that lead to improved performance. Although it is well established that the basal ganglia (BG) process signals relating to action evaluation and selection, whether and how the BG promote exploratory motor variability remain unclear. We investigated this question in songbirds, which learn to produce complex vocalizations through trial and error. In contrast with previous studies that did not find effects of BG lesions on vocal motor variability, we demonstrate that the BG are an essential source of rapid behavioral variation linked to vocal learning.
Keywords: basal ganglia; motor exploration; reinforcement learning; social context; songbird; vocal learning.
Copyright © 2018 the authors 0270-6474/18/389635-13$15.00/0.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
