Interactive Repression of MYRF Self-Cleavage and Activity in Oligodendrocyte Differentiation by TMEM98 Protein
- PMID: 30249802
- PMCID: PMC6596239
- DOI: 10.1523/JNEUROSCI.0154-18.2018
Interactive Repression of MYRF Self-Cleavage and Activity in Oligodendrocyte Differentiation by TMEM98 Protein
Abstract
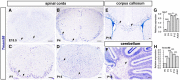
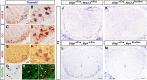
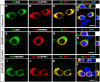
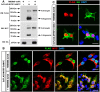
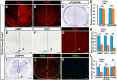
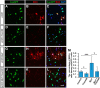
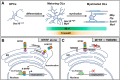
Myelin sheath formed by oligodendrocytes (OLs) is essential for the rapid propagation of action potentials in the vertebrate CNS. Myelin regulatory factor (MYRF) is one of the critical factors that control OL differentiation and myelin maintenance. Previous studies showed that MYRF is a membrane-bound transcription factor associated with the endoplasmic reticulum (ER). After self-cleavage, the N-fragment of MYRF is released from the ER and translocated into the nucleus where it functions as a transcription factor to activate myelin gene expression. At present, it remains unknown whether MYRF self-cleavage and functional activation can be regulated during OL differentiation. Here, we report that TMEM98, an ER-associated transmembrane protein, is capable of binding to the C-terminal of MYRF and inhibiting its self-cleavage and N-fragment nuclear translocation. In the developing CNS, TMEM98 is selectively expressed in early maturing OLs in mouse pups of either sex. Forced expression of TMEM98 in embryonic chicken spinal cord of either sex suppresses endogenous OL differentiation and MYRF-induced ectopic expression of myelin genes. These results suggest that TMEM98, through inhibiting the self-cleavage of MYRF, functions as a negative feedback regulator of MYRF in oligodendrocyte differentiation and myelination.SIGNIFICANCE STATEMENT MYRF protein is initially synthesized as an ER-associated membrane protein that undergoes autoproteolytic cleavage to release the N-fragment, which is then transported into the nucleus and activates the transcription of myelin genes. To date, the molecular mechanisms that regulate the self-cleavage and function of MYRF in regulating oligodendrocyte differentiation have remained unknown. In this study, we present the molecular and functional evidence that TMEM98 membrane protein physically interacts with MYRF in the ER and subsequently blocks its self-cleavage, N-terminal nuclear translocation, and functional activation of myelin gene expression. To our knowledge, this is the first report on the regulation of MYRF self-proteolytic activity and function by an interacting protein, providing new insights into the molecular regulation of OL differentiation and myelinogenesis.
Keywords: MYRF; TMEM98; oligodendrocyte differentiation; self-cleavage.
Copyright © 2018 the authors 0270-6474/18/389829-11$15.00/0.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
