Pioglitazone Protects Mesenchymal Stem Cells against P-Cresol-Induced Mitochondrial Dysfunction via Up-Regulation of PINK-1
- PMID: 30250007
- PMCID: PMC6213327
- DOI: 10.3390/ijms19102898
Pioglitazone Protects Mesenchymal Stem Cells against P-Cresol-Induced Mitochondrial Dysfunction via Up-Regulation of PINK-1
Abstract
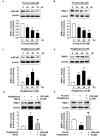
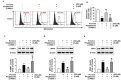
Mesenchymal stem cells (MSC) could be a candidate for cell-based therapy in chronic kidney disease (CKD); however, the uremic toxin in patients with CKD restricts the therapeutic efficacy of MSCs. To address this problem, we explored the effect of pioglitazone as a measure against exposure to the uremic toxin P-cresol (PC) in MSCs. Under PC exposure conditions, apoptosis of MSCs was induced, as well as PC-induced dysfunction of mitochondria by augmentation of mitofusion, reduction of mitophagy, and inactivation of mitochondrial complexes I and IV. Treatment of MSCs with pioglitazone significantly inhibited PC-induced apoptosis. Pioglitazone also prevented PC-induced mitofusion and increased mitophagy against PC exposure through up-regulation of phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK-1). Furthermore, pioglitazone protected against PC-induced mitochondrial dysfunction by increasing the cytochrome c oxidase subunit 4 (COX4) level and activating complexes I and IV, resulting in enhancement of proliferation. In particular, activation of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) regulated the pioglitazone-mediated up-regulation of PINK-1. These results indicate that pioglitazone protects MSCs against PC-induced accumulated mitochondrial dysfunction via the NF-κB⁻PINK-1 axis under P-cresol exposure conditions. Our study suggests that pioglitazone-treated MSCs could be a candidate for MSC-based therapy in patients with CKD.
Keywords: PTEN-induced putative kinase 1; cell proliferation; chronic kidney disease; mesenchymal stem cell; pioglitazone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Pioglitazone Improves the Function of Human Mesenchymal Stem Cells in Chronic Kidney Disease Patients.Int J Mol Sci. 2019 May 10;20(9):2314. doi: 10.3390/ijms20092314. Int J Mol Sci. 2019. PMID: 31083336 Free PMC article.
-
Fucoidan Rescues p-Cresol-Induced Cellular Senescence in Mesenchymal Stem Cells via FAK-Akt-TWIST Axis.Mar Drugs. 2018 Apr 6;16(4):121. doi: 10.3390/md16040121. Mar Drugs. 2018. PMID: 29642406 Free PMC article.
-
Co-Administration of Melatonin Effectively Enhances the Therapeutic Effects of Pioglitazone on Mesenchymal Stem Cells Undergoing Indoxyl Sulfate-Induced Senescence through Modulation of Cellular Prion Protein Expression.Int J Mol Sci. 2018 May 4;19(5):1367. doi: 10.3390/ijms19051367. Int J Mol Sci. 2018. PMID: 29734669 Free PMC article.
-
Mesenchymal stem cells and their mitochondrial transfer: a double-edged sword.Biosci Rep. 2019 May 3;39(5):BSR20182417. doi: 10.1042/BSR20182417. Print 2019 May 31. Biosci Rep. 2019. PMID: 30979829 Free PMC article. Review.
-
Mesenchymal stem cell therapy targeting mitochondrial dysfunction in acute kidney injury.J Transl Med. 2019 May 2;17(1):142. doi: 10.1186/s12967-019-1893-4. J Transl Med. 2019. PMID: 31046805 Free PMC article. Review.
Cited by
-
Development of Cell Therapies for Renal Disease and Regenerative Medicine.Int J Mol Sci. 2022 Dec 15;23(24):15943. doi: 10.3390/ijms232415943. Int J Mol Sci. 2022. PMID: 36555585 Free PMC article. Review.
-
PTEN-induced kinase 1 enhances the reparative effects of bone marrow mesenchymal stromal cells on mice with renal ischaemia/reperfusion-induced acute kidney injury.Hum Cell. 2022 Nov;35(6):1650-1670. doi: 10.1007/s13577-022-00756-8. Epub 2022 Aug 12. Hum Cell. 2022. PMID: 35962179 Free PMC article.
-
Effects of p-Cresol on Senescence, Survival, Inflammation, and Odontoblast Differentiation in Canine Dental Pulp Stem Cells.Int J Mol Sci. 2020 Sep 21;21(18):6931. doi: 10.3390/ijms21186931. Int J Mol Sci. 2020. PMID: 32967298 Free PMC article.
-
TUDCA-Treated Mesenchymal Stem Cells Protect against ER Stress in the Hippocampus of a Murine Chronic Kidney Disease Model.Int J Mol Sci. 2019 Jan 31;20(3):613. doi: 10.3390/ijms20030613. Int J Mol Sci. 2019. PMID: 30708974 Free PMC article.
-
Potential and Therapeutic Efficacy of Cell-based Therapy Using Mesenchymal Stem Cells for Acute/chronic Kidney Disease.Int J Mol Sci. 2019 Apr 1;20(7):1619. doi: 10.3390/ijms20071619. Int J Mol Sci. 2019. PMID: 30939749 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

