Characterization of Properties and Transglycosylation Abilities of Recombinant α-Galactosidase from Cold-Adapted Marine Bacterium Pseudoalteromonas KMM 701 and Its C494N and D451A Mutants
- PMID: 30250010
- PMCID: PMC6213131
- DOI: 10.3390/md16100349
Characterization of Properties and Transglycosylation Abilities of Recombinant α-Galactosidase from Cold-Adapted Marine Bacterium Pseudoalteromonas KMM 701 and Its C494N and D451A Mutants
Abstract
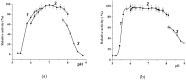
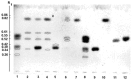
A novel wild-type recombinant cold-active α-d-galactosidase (α-PsGal) from the cold-adapted marine bacterium Pseudoalteromonas sp. KMM 701, and its mutants D451A and C494N, were studied in terms of their structural, physicochemical, and catalytic properties. Homology models of the three-dimensional α-PsGal structure, its active center, and complexes with D-galactose were constructed for identification of functionally important amino acid residues in the active site of the enzyme, using the crystal structure of the α-galactosidase from Lactobacillus acidophilus as a template. The circular dichroism spectra of the wild α-PsGal and mutant C494N were approximately identical. The C494N mutation decreased the efficiency of retaining the affinity of the enzyme to standard p-nitrophenyl-α-galactopiranoside (pNP-α-Gal). Thin-layer chromatography, matrix-assisted laser desorption/ionization mass spectrometry, and nuclear magnetic resonance spectroscopy methods were used to identify transglycosylation products in reaction mixtures. α-PsGal possessed a narrow acceptor specificity. Fructose, xylose, fucose, and glucose were inactive as acceptors in the transglycosylation reaction. α-PsGal synthesized -α(1→6)- and -α(1→4)-linked galactobiosides from melibiose as well as -α(1→6)- and -α(1→3)-linked p-nitrophenyl-digalactosides (Gal₂-pNP) from pNP-α-Gal. The D451A mutation in the active center completely inactivated the enzyme. However, the substitution of C494N discontinued the Gal-α(1→3)-Gal-pNP synthesis and increased the Gal-α(1→4)-Gal yield compared to Gal-α(1→6)-Gal-pNP.
Keywords: GH 36 family; Pseudoalteromonas sp. KMM 701; homology model; marine bacteria; mutation; transglycosylation; α-d-galactosidase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Optimization of cold-adapted alpha-galactosidase expression in Escherichia coli.Protein Expr Purif. 2016 Jul;123:14-8. doi: 10.1016/j.pep.2016.03.006. Epub 2016 Mar 23. Protein Expr Purif. 2016. PMID: 27033343
-
Characterization and site-directed mutagenesis of an α-galactosidase from the deep-sea bacterium Bacillus megaterium.Enzyme Microb Technol. 2014 Mar 5;56:46-52. doi: 10.1016/j.enzmictec.2014.01.004. Epub 2014 Jan 16. Enzyme Microb Technol. 2014. PMID: 24564902
-
Increasing the transglycosylation activity of alpha-galactosidase from Bifidobacterium adolescentis DSM 20083 by site-directed mutagenesis.Biotechnol Bioeng. 2006 Jan 5;93(1):122-31. doi: 10.1002/bit.20713. Biotechnol Bioeng. 2006. PMID: 16320365
-
Improvement of the efficiency of transglycosylation catalyzed by α-galactosidase from Thermotoga maritima by protein engineering.Biochemistry (Mosc). 2013 Oct;78(10):1112-23. doi: 10.1134/S0006297913100052. Biochemistry (Mosc). 2013. PMID: 24237145
-
Molecular Characterization of the α-Galactosidase SCO0284 from Streptomyces coelicolor A3(2), a Family 27 Glycosyl Hydrolase.J Microbiol Biotechnol. 2016 Sep 28;26(9):1650-6. doi: 10.4014/jmb.1606.06010. J Microbiol Biotechnol. 2016. PMID: 27363469
Cited by
-
Deep-Sea Anemones Are Prospective Source of New Antimicrobial and Cytotoxic Compounds.Mar Drugs. 2021 Nov 24;19(12):654. doi: 10.3390/md19120654. Mar Drugs. 2021. PMID: 34940653 Free PMC article.
-
Effect of Pentacyclic Guanidine Alkaloids from the Sponge Monanchora pulchra on Activity of α-Glycosidases from Marine Bacteria.Mar Drugs. 2019 Jan 1;17(1):22. doi: 10.3390/md17010022. Mar Drugs. 2019. PMID: 30609674 Free PMC article.
-
Genomic Features of a Food-Derived Pseudomonas aeruginosa Strain PAEM and Biofilm-Associated Gene Expression under a Marine Bacterial α-Galactosidase.Int J Mol Sci. 2020 Oct 16;21(20):7666. doi: 10.3390/ijms21207666. Int J Mol Sci. 2020. PMID: 33081309 Free PMC article.
-
Effects of Sponge-Derived Alkaloids on Activities of the Bacterial α-D-Galactosidase and Human Cancer Cell α-N-Acetylgalactosaminidase.Biomedicines. 2021 May 5;9(5):510. doi: 10.3390/biomedicines9050510. Biomedicines. 2021. PMID: 34063022 Free PMC article.
-
Biocatalysis of Fucodian in Undaria pinnatifida Sporophyll Using Bifidobacterium longum RD47 for Production of Prebiotic Fucosylated Oligosaccharide.Mar Drugs. 2019 Feb 14;17(2):117. doi: 10.3390/md17020117. Mar Drugs. 2019. PMID: 30769784 Free PMC article.
References
-
- Koshland D.E., Jr. Stereochemistry and the mechanism of enzymatic reactions. Biol. Rev. Camb. Phil. Soc. 1953;28:416–436. doi: 10.1111/j.1469-185X.1953.tb01386.x. - DOI
-
- Ivanova E.P., Bakunina I.Y., Nedashkovskaya O.I., Gorshkova N.M., Mikhailov V.V., Elyakova L.A. Incidence of marine microorganisms producing β-N-acetylglucosaminidases, α-d-galactosidases and α-N-acetylgalactosaminidases. Rus. J. Mar. Biol. 1998;24:365–372.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

