Rapamycin and fasting sustain autophagy response activated by ischemia/reperfusion injury and promote retinal ganglion cell survival
- PMID: 30250019
- PMCID: PMC6155349
- DOI: 10.1038/s41419-018-1044-5
Rapamycin and fasting sustain autophagy response activated by ischemia/reperfusion injury and promote retinal ganglion cell survival
Abstract
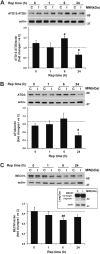
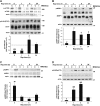
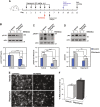
Autophagy, the cellular process responsible for degradation and recycling of cytoplasmic components through the autophagosomal-lysosomal pathway, is fundamental for neuronal homeostasis and its deregulation has been identified as a hallmark of neurodegeneration. Retinal hypoxic-ischemic events occur in several sight-treating disorders, such as central retinal artery occlusion, diabetic retinopathy, and glaucoma, leading to degeneration and loss of retinal ganglion cells. Here we analyzed the autophagic response in the retinas of mice subjected to ischemia induced by transient elevation of intraocular pressure, reporting a biphasic and reperfusion time-dependent modulation of the process. Ischemic insult triggered in the retina an acute induction of autophagy that lasted during the first hours of reperfusion. This early upregulation of the autophagic flux limited RGC death, as demonstrated by the increased neuronal loss observed in mice with genetic impairment of basal autophagy owing to heterozygous ablation of the autophagy-positive modulator Ambra1 (Ambra1+/gt). Upregulation of autophagy was exhausted 24 h after the ischemic event and reduced autophagosomal turnover was associated with build up of the autophagic substrate SQSTM-1/p62, decreased ATG12-ATG5 conjugate, ATG4 and BECN1/Beclin1 expression. Animal fasting or subchronic systemic treatment with rapamycin sustained and prolonged autophagy activation and improved RGC survival, providing proof of principle for autophagy induction as a potential therapeutic strategy in retinal neurodegenerative conditions associated with hypoxic/ischemic stresses.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

