Skin α-synuclein deposits differ in clinical variants of synucleinopathy: an in vivo study
- PMID: 30250046
- PMCID: PMC6155202
- DOI: 10.1038/s41598-018-32588-8
Skin α-synuclein deposits differ in clinical variants of synucleinopathy: an in vivo study
Abstract
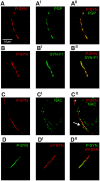
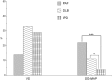
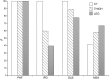
We aimed to characterize in vivo α-synuclein (α-syn) aggregates in skin nerves to ascertain: 1) the optimal marker to identify them; 2) possible differences between synucleinopathies that may justify the clinical variability. We studied multiple skin nerve α-syn deposits in 44 patients with synucleinopathy: 15 idiopathic Parkinson's disease (IPD), 12 dementia with Lewy Bodies (DLB), 5 pure autonomic failure (PAF) and 12 multiple system atrophy (MSA). Ten healthy subjects were used as controls. Antibodies against native α-syn, C-terminal α-syn epitopes such as phosphorylation at serine 129 (p-syn) and to conformation-specific for α-syn mature amyloid fibrils (syn-F1) were used. We found that p-syn showed the highest sensitivity and specificity in disclosing skin α-syn deposits. In MSA abnormal deposits were only found in somatic fibers mainly at distal sites differently from PAF, IPD and DLB displaying α-syn deposits in autonomic fibers mainly at proximal sites. PAF and DLB showed the highest p-syn load with a widespread involvement of autonomic skin nerve fibers.
In conclusion: 1) p-syn in skin nerves was the optimal marker for the in vivo diagnosis of synucleinopathies; 2) the localization and load differences of aggregates may help to identify specific diagnostic traits and support a different pathogenesis among synucleinopathies.
Conflict of interest statement
The authors declare no competing interests.
Figures


Comment in
-
A novel autosomal recessive orthostatic hypotension syndrome: and other updates on recent autonomic research.Clin Auton Res. 2018 Dec;28(6):565-567. doi: 10.1007/s10286-018-0578-z. Epub 2018 Nov 10. Clin Auton Res. 2018. PMID: 30415401 No abstract available.
Similar articles
-
Comparison of 123I-MIBG scintigraphy and phosphorylated α-synuclein skin deposits in synucleinopathies.Parkinsonism Relat Disord. 2020 Dec;81:48-53. doi: 10.1016/j.parkreldis.2020.10.016. Epub 2020 Oct 8. Parkinsonism Relat Disord. 2020. PMID: 33049589
-
Skin nerve misfolded α-synuclein in pure autonomic failure and Parkinson disease.Ann Neurol. 2016 Feb;79(2):306-16. doi: 10.1002/ana.24567. Epub 2015 Dec 29. Ann Neurol. 2016. PMID: 26606657
-
Pathophysiological Significance of α-Synuclein in Sympathetic Nerves: In Vivo Observations.Neurology. 2025 Feb 11;104(3):e210215. doi: 10.1212/WNL.0000000000210215. Epub 2025 Jan 13. Neurology. 2025. PMID: 39805051
-
Neuropathology underlying clinical variability in patients with synucleinopathies.Acta Neuropathol. 2011 Aug;122(2):187-204. doi: 10.1007/s00401-011-0852-9. Epub 2011 Jul 1. Acta Neuropathol. 2011. PMID: 21720849 Review.
-
ɑ-Synuclein strains and the variable pathologies of synucleinopathies.J Neurochem. 2016 Oct;139 Suppl 1:256-274. doi: 10.1111/jnc.13595. Epub 2016 Mar 30. J Neurochem. 2016. PMID: 26924014 Review.
Cited by
-
Skin nerve phosphorylated α-synuclein in the elderly.J Neuropathol Exp Neurol. 2024 Mar 20;83(4):245-250. doi: 10.1093/jnen/nlae015. J Neuropathol Exp Neurol. 2024. PMID: 38408377 Free PMC article.
-
Skin Conditions and Movement Disorders: Hiding in Plain Sight.Mov Disord Clin Pract. 2022 Mar 24;9(5):566-583. doi: 10.1002/mdc3.13436. eCollection 2022 Jul. Mov Disord Clin Pract. 2022. PMID: 35844274 Free PMC article. Review.
-
Evaluating the Diagnostic Potential of Combined Salivary and Skin Biomarkers in Parkinson's Disease.Int J Mol Sci. 2024 Apr 28;25(9):4823. doi: 10.3390/ijms25094823. Int J Mol Sci. 2024. PMID: 38732041 Free PMC article.
-
Peripheral synucleinopathy in Parkinson disease with LRRK2 G2385R variants.Ann Clin Transl Neurol. 2021 Mar;8(3):592-602. doi: 10.1002/acn3.51301. Epub 2021 Feb 1. Ann Clin Transl Neurol. 2021. PMID: 33527742 Free PMC article.
-
Posttranslational Modifications of α-Synuclein, Their Therapeutic Potential, and Crosstalk in Health and Neurodegenerative Diseases.Pharmacol Rev. 2024 Oct 16;76(6):1254-1290. doi: 10.1124/pharmrev.123.001111. Pharmacol Rev. 2024. PMID: 39164116 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

