Biallelic mutations in FDXR cause neurodegeneration associated with inflammation
- PMID: 30250212
- PMCID: PMC6451867
- DOI: 10.1038/s10038-018-0515-y
Biallelic mutations in FDXR cause neurodegeneration associated with inflammation
Abstract
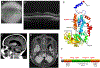
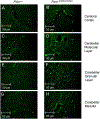
Mitochondrial dysfunction lies behind many neurodegenerative disorders, owing largely to the intense energy requirements of most neurons. Such mitochondrial dysfunction may work through a variety of mechanisms, from direct disruption of the electron transport chain to abnormal mitochondrial biogenesis. Recently, we have identified biallelic mutations in the mitochondrial flavoprotein "ferredoxin reductase" (FDXR) gene as a novel cause of mitochondriopathy, peripheral neuropathy, and optic atrophy. In this report, we expand upon those results by describing two new cases of disease-causing FDXR variants in patients with variable severity of phenotypes, including evidence of an inflammatory response in brain autopsy. To investigate the underlying pathogenesis, we examined neurodegeneration in a mouse model. We found that Fdxr mutant mouse brain tissues share pathological changes similar to those seen in patient autopsy material, including increased astrocytes. Furthermore, we show that these abnormalities are associated with increased levels of markers for both neurodegeneration and gliosis, with the latter implying inflammation as a major factor in the pathology of Fdxr mutations. These data provide further insight into the pathogenic mechanism of FDXR-mediated central neuropathy, and suggest an avenue for mechanistic studies that will ultimately inform treatment.
Conflict of interest statement
Figures




References
-
- Shoffner JM, Lott MT, Lezza AMS, Seibel P, Ballinger SW, Wallace DC. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNALys mutation. Cell. 1990;61(6):931–7. - PubMed
-
- Nijtmans LG, Henderson NS, Attardi G, Holt IJ. Impaired ATP synthase assembly associated with a mutation in the human ATP synthase subunit 6 gene. The Journal of biological chemistry. 2001;276(9):6755–62. - PubMed
-
- Goto Y, Nonaka I, Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348(6302):651–3. - PubMed
-
- Webert H, Freibert SA, Gallo A, Heidenreich T, Linne U, Amlacher S, et al. Functional reconstitution of mitochondrial Fe/S cluster synthesis on Isu1 reveals the involvement of ferredoxin. Nature communications. 2014;5:5013. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
