The interaction landscape between transcription factors and the nucleosome
- PMID: 30250250
- PMCID: PMC6173309
- DOI: 10.1038/s41586-018-0549-5
The interaction landscape between transcription factors and the nucleosome
Abstract
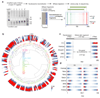
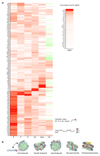
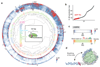
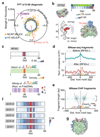
Nucleosomes cover most of the genome and are thought to be displaced by transcription factors in regions that direct gene expression. However, the modes of interaction between transcription factors and nucleosomal DNA remain largely unknown. Here we systematically explore interactions between the nucleosome and 220 transcription factors representing diverse structural families. Consistent with earlier observations, we find that the majority of the studied transcription factors have less access to nucleosomal DNA than to free DNA. The motifs recovered from transcription factors bound to nucleosomal and free DNA are generally similar. However, steric hindrance and scaffolding by the nucleosome result in specific positioning and orientation of the motifs. Many transcription factors preferentially bind close to the end of nucleosomal DNA, or to periodic positions on the solvent-exposed side of the DNA. In addition, several transcription factors usually bind to nucleosomal DNA in a particular orientation. Some transcription factors specifically interact with DNA located at the dyad position at which only one DNA gyre is wound, whereas other transcription factors prefer sites spanning two DNA gyres and bind specifically to each of them. Our work reveals notable differences in the binding of transcription factors to free and nucleosomal DNA, and uncovers a diverse interaction landscape between transcription factors and the nucleosome.
Conflict of interest statement
The authors declare no competing financial interests.
Figures










Comment in
-
Spotlight on nucleosomes.Nat Rev Genet. 2018 Dec;19(12):738-739. doi: 10.1038/s41576-018-0070-6. Nat Rev Genet. 2018. PMID: 30367164 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

