Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases
- PMID: 30250260
- PMCID: PMC6432913
- DOI: 10.1038/s41593-018-0235-9
Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases
Abstract
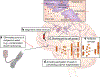
A hallmark event in neurodegenerative diseases (NDs) is the misfolding, aggregation, and accumulation of proteins, leading to cellular dysfunction, loss of synaptic connections, and brain damage. Despite the involvement of distinct proteins in different NDs, the process of protein misfolding and aggregation is remarkably similar. A recent breakthrough in the field was the discovery that misfolded protein aggregates can self-propagate through seeding and spread the pathological abnormalities between cells and tissues in a manner akin to the behavior of infectious prions in prion diseases. This discovery has vast implications for understanding the mechanisms involved in the initiation and progression of NDs, as well as for the design of novel strategies for treatment and diagnosis. In this Review, we provide a critical discussion of the role of protein misfolding and aggregation in NDs. Commonalities and differences between distinct protein aggregates will be highlighted, in addition to evidence supporting the hypothesis that misfolded aggregates can be transmissible by the prion principle. We will also describe the molecular basis and implications for prion-like conformational strains, cross-interaction between different misfolded proteins in the brain, and how these concepts can be applied to the development of novel strategies for therapy and diagnosis.
Conflict of interest statement
Competing interests
C.S. is the inventor of the PMCA technology and is currently the Founder, Chief Scientific Officer, and major shareholder of Amprion Inc., a biotech company aiming to develop PMCA and RT-QuIC seeding amplification assays for diagnosis of neurodegenerative diseases.
Figures

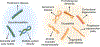
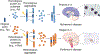
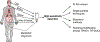
References
-
- Ross CA & Poirier MA Protein aggregation and neurodegenerative disease. Nat. Med 10(Suppl), S10–S17 (2004). - PubMed
-
- Soto C Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci 4, 49–60 (2003). - PubMed
-
- Goedert M Neurodegeneration. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 349, 1255555 (2015). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

