The role of brain vasculature in neurodegenerative disorders
- PMID: 30250261
- PMCID: PMC6198802
- DOI: 10.1038/s41593-018-0234-x
The role of brain vasculature in neurodegenerative disorders
Abstract
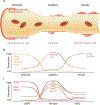
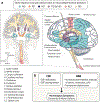
Adequate supply of blood and structural and functional integrity of blood vessels are key to normal brain functioning. On the other hand, cerebral blood flow shortfalls and blood-brain barrier dysfunction are early findings in neurodegenerative disorders in humans and animal models. Here we first examine molecular definition of cerebral blood vessels, as well as pathways regulating cerebral blood flow and blood-brain barrier integrity. Then we examine the role of cerebral blood flow and blood-brain barrier in the pathogenesis of Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis, and multiple sclerosis. We focus on Alzheimer's disease as a platform of our analysis because more is known about neurovascular dysfunction in this disease than in other neurodegenerative disorders. Finally, we propose a hypothetical model of Alzheimer's disease biomarkers to include brain vasculature as a factor contributing to the disease onset and progression, and we suggest a common pathway linking brain vascular contributions to neurodegeneration in multiple neurodegenerative disorders.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

