Atrial Innervation Patterns of Intrinsic Cardiac Autonomic Nerves
- PMID: 30250413
- PMCID: PMC6146147
- DOI: 10.3346/jkms.2018.33.e253
Atrial Innervation Patterns of Intrinsic Cardiac Autonomic Nerves
Abstract
Background: Although ganglionated plexi (GPs) are important in the pathogenesis of arrhythmia, their patterns of atrial innervation have remained unclear. We investigated patterns of GP innervation to cardiac atria and the neuroanatomical interconnections among GPs in an animal model.
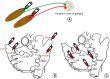
Methods: Atrial innervation by GPs was evaluated in 10 mongrel dogs using a retrograde neuronal tracer (cholera toxin subunit B [CTB] conjugated with fluorescent dyes). In Experiment 1, CTB was injected into the atria. In Experiment 2, CTB was injected into the major GP, including the anterior right GP (ARGP), inferior right GP (IRGP), superior left GP (SLGP), and ligament of Marshall (LOM). After 7 days, the GPs were examined for the presence of tracer-positive neurons.
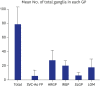
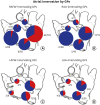
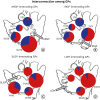
Results: GPs in either right or left-side were innervating to both the same and opposite sides of the atrium. In quantitative analysis, right-sided GPs, especially ARGP, showed numerical predominance in atrial innervation. Based on the proportion of CTB-labeled ganglion in each GP, atrial innervation by GPs showed a tendency of laterality. In Experiment 2, CTB that was injected to a particular GP widely distributed in different GP. ARGP projected the largest number of innervating neurons to the IRGP, SLGP and LOM.
Conclusion: This study demonstrated that GPs project axons widely to both the same and opposite sides of atria. ARGP played a dominant role in atrial innervation. Furthermore, there were numerous neuroanatomical interconnections among GPs. These findings about neuronal innervation and interconnections of GPs could offer useful information for understanding intrinsic cardiac nervous system neuroanatomy.
Keywords: Atrium; Autonomic Nervous System; Ganglionated Plexi; Innervation; Mongrel Dogs.
Conflict of interest statement
Disclosure: The authors have no potential conflicts of interest to disclose.
Figures


Similar articles
-
Autonomic elements within the ligament of Marshall and inferior left ganglionated plexus mediate functions of the atrial neural network.J Cardiovasc Electrophysiol. 2009 Mar;20(3):318-24. doi: 10.1111/j.1540-8167.2008.01315.x. J Cardiovasc Electrophysiol. 2009. PMID: 19261040
-
Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation.J Am Coll Cardiol. 2007 Jul 3;50(1):61-8. doi: 10.1016/j.jacc.2007.02.066. Epub 2007 Jun 18. J Am Coll Cardiol. 2007. PMID: 17601547
-
Autonomic mechanism for initiation of rapid firing from atria and pulmonary veins: evidence by ablation of ganglionated plexi.Cardiovasc Res. 2009 Nov 1;84(2):245-52. doi: 10.1093/cvr/cvp194. Epub 2009 Jun 11. Cardiovasc Res. 2009. PMID: 19520703 Free PMC article.
-
Ganglionated plexi as neuromodulation targets for atrial fibrillation.J Cardiovasc Electrophysiol. 2017 Dec;28(12):1485-1491. doi: 10.1111/jce.13319. Epub 2017 Sep 8. J Cardiovasc Electrophysiol. 2017. PMID: 28833764 Free PMC article. Review.
-
Is the Atrial Neural Plexis a Therapeutic Target in Atrial Fibrillation?Methodist Debakey Cardiovasc J. 2015 Apr-Jun;11(2):82-6. doi: 10.14797/mdcj-11-2-82. Methodist Debakey Cardiovasc J. 2015. PMID: 26306124 Free PMC article. Review.
Cited by
-
Neuromodulation for Atrial Fibrillation Control.Korean Circ J. 2024 May;54(5):223-232. doi: 10.4070/kcj.2024.0050. Epub 2024 Mar 18. Korean Circ J. 2024. PMID: 38654454 Free PMC article. Review.
-
Extracardiac Vagal Stimulation-Assisted Cardioneuroablation: Dynamically Evaluating the Impact of Sequential Ganglionated Plexus Ablation on Vagal Control of SAN and AVN in Patients with Sinoatrial Node Dysfunction.J Cardiovasc Dev Dis. 2022 Jun 10;9(6):188. doi: 10.3390/jcdd9060188. J Cardiovasc Dev Dis. 2022. PMID: 35735817 Free PMC article.
References
-
- Scherlag BJ, Yamanashi W, Patel U, Lazzara R, Jackman WM. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J Am Coll Cardiol. 2005;45(11):1878–1886. - PubMed
-
- Yuan BX, Ardell JL, Hopkins DA, Losier AM, Armour JA. Gross and microscopic anatomy of the canine intrinsic cardiac nervous system. Anat Rec. 1994;239(1):75–87. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

