Human Endogenous Retroviruses Are Ancient Acquired Elements Still Shaping Innate Immune Responses
- PMID: 30250470
- PMCID: PMC6139349
- DOI: 10.3389/fimmu.2018.02039
Human Endogenous Retroviruses Are Ancient Acquired Elements Still Shaping Innate Immune Responses
Abstract
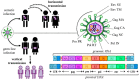
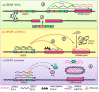
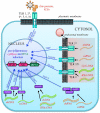
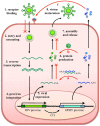
About 8% of our genome is composed of sequences with viral origin, namely human Endogenous Retroviruses (HERVs). HERVs are relics of ancient infections that affected the primates' germ line along the last 100 million of years, and became stable elements at the interface between self and foreign DNA. Intriguingly, HERV co-evolution with the host led to the domestication of activities previously devoted to the retrovirus life cycle, providing novel cellular functions. For example, selected HERV envelope proteins have been coopted for pregnancy-related purposes, and proviral Long Terminal Repeats participate in the transcriptional regulation of various cellular genes. Given the HERV persistence in the host genome and its basal expression in most healthy tissues, it is reasonable that human defenses should prevent HERV-mediated immune activation. Despite this, HERVs and their products (including RNA, cytosolic DNA, and proteins) are still able to modulate and be influenced by the host immune system, fascinatingly suggesting a central role in the evolution and functioning of the human innate immunity. Indeed, HERV sequences had been major contributors in shaping and expanding the interferon network, dispersing inducible genes that have been occasionally domesticated in various mammalian lineages. Also the HERV integration within or near to genes encoding for critical immune factors has been shown to influence their activity, or to be responsible for their polymorphic variation in the human population, such as in the case of an HERV-K(HML10) provirus in the major histocompatibility complex region. In addition, HERV expressed products have been shown to modulate innate immunity effectors, being therefore often related on the one side to inflammatory and autoimmune disorders, while on the other side to the control of excessive immune activation through their immunosuppressive properties. Finally, HERVs have been proposed to establish a protective effect against exogenous infections. The present review summarizes the involvement of HERVs and their products in innate immune responses, describing how their intricate interplay with the first line of human defenses can actively contribute either to the host protection or to his damage, implying a subtle balance between the persistence of HERV expression and the maintenance of a basal immune alert.
Keywords: HERV; autoimmunity; cancer; endogenous retroviruses; evolution; innate immunity; interferon.
Figures
Similar articles
-
Human endogenous retroviruses and cancer prevention: evidence and prospects.BMC Cancer. 2013 Jan 3;13:4. doi: 10.1186/1471-2407-13-4. BMC Cancer. 2013. PMID: 23282240 Free PMC article.
-
Type W Human Endogenous Retrovirus (HERV-W) Integrations and Their Mobilization by L1 Machinery: Contribution to the Human Transcriptome and Impact on the Host Physiopathology.Viruses. 2017 Jun 27;9(7):162. doi: 10.3390/v9070162. Viruses. 2017. PMID: 28653997 Free PMC article. Review.
-
Human endogenous retrovirus HERV-K14 families: status, variants, evolution, and mobilization of other cellular sequences.J Virol. 2005 Mar;79(5):2941-9. doi: 10.1128/JVI.79.5.2941-2949.2005. J Virol. 2005. PMID: 15709013 Free PMC article.
-
HERV-W group evolutionary history in non-human primates: characterization of ERV-W orthologs in Catarrhini and related ERV groups in Platyrrhini.BMC Evol Biol. 2018 Jan 19;18(1):6. doi: 10.1186/s12862-018-1125-1. BMC Evol Biol. 2018. PMID: 29351742 Free PMC article.
-
Retroviruses and primate evolution.Bioessays. 2000 Feb;22(2):161-71. doi: 10.1002/(SICI)1521-1878(200002)22:2<161::AID-BIES7>3.0.CO;2-X. Bioessays. 2000. PMID: 10655035 Review.
Cited by
-
Human Endogenous Retroviruses in Clear Cell Renal Cell Carcinoma: Biological Functions and Clinical Values.Onco Targets Ther. 2020 Aug 7;13:7877-7885. doi: 10.2147/OTT.S259534. eCollection 2020. Onco Targets Ther. 2020. PMID: 32821127 Free PMC article. Review.
-
Long-term host-pathogen evolution of endogenous beta- and gammaretroviruses in mouse lemurs with little evidence of recent retroviral introgression.Virus Evol. 2022 Dec 14;9(1):veac117. doi: 10.1093/ve/veac117. eCollection 2023. Virus Evol. 2022. PMID: 36632481 Free PMC article.
-
Significant Upregulation of HERV-K (HML-2) Transcription Levels in Human Lung Cancer and Cancer Cells.Front Microbiol. 2022 Mar 10;13:850444. doi: 10.3389/fmicb.2022.850444. eCollection 2022. Front Microbiol. 2022. PMID: 35359739 Free PMC article.
-
Ancient dormant virus remnant ERVW-1 drives ferroptosis via degradation of GPX4 and SLC3A2 in schizophrenia.Virol Sin. 2024 Feb;39(1):31-43. doi: 10.1016/j.virs.2023.09.001. Epub 2023 Sep 9. Virol Sin. 2024. PMID: 37690733 Free PMC article.
-
Microorganisms as Shapers of Human Civilization, from Pandemics to Even Our Genomes: Villains or Friends? A Historical Approach.Microorganisms. 2021 Dec 6;9(12):2518. doi: 10.3390/microorganisms9122518. Microorganisms. 2021. PMID: 34946123 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

