Therapeutic Potential of the Gut Microbiota in the Prevention and Treatment of Sepsis
- PMID: 30250472
- PMCID: PMC6139316
- DOI: 10.3389/fimmu.2018.02042
Therapeutic Potential of the Gut Microbiota in the Prevention and Treatment of Sepsis
Abstract
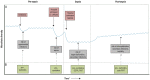
Alongside advances in understanding the pathophysiology of sepsis, there have been tremendous strides in understanding the pervasive role of the gut microbiota in systemic host resistance. In pre-clinical models, a diverse and balanced gut microbiota enhances host immunity to both enteric and systemic pathogens. Disturbance of this balance increases susceptibility to sepsis and sepsis-related organ dysfunction, while restoration of the gut microbiome is protective. Patients with sepsis have a profoundly distorted composition of the intestinal microbiota, but the impact and therapeutic potential of the microbiome is not well-established in human sepsis. Modulation of the microbiota consists of either resupplying the pool of beneficial microbes by administration of probiotics, improving the intestinal microenvironment to enhance the growth of beneficial species by dietary interventions and prebiotics, or by totally recolonizing the gut with a fecal microbiota transplantation (FMT). We propose that there are three potential opportunities to utilize these treatment modalities over the course of sepsis: to decrease sepsis incidence, to improve sepsis outcome, and to decrease late mortality after sepsis. Exploring these three avenues will provide insight into how disturbances of the microbiota can predispose to, or even perpetuate the dysregulated immune response associated with this syndrome, which in turn could be associated with improved sepsis management.
Keywords: fecal microbiota transplantation; microbiota; pathogenesis; probiotics and synbiotics; sepsis; therapeutics.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

