Smokeless tobacco extract inhibits proliferation and promotes apoptosis in oral mucous fibroblasts
- PMID: 30250574
- PMCID: PMC6144942
- DOI: 10.3892/ol.2018.9252
Smokeless tobacco extract inhibits proliferation and promotes apoptosis in oral mucous fibroblasts
Abstract
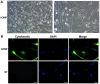
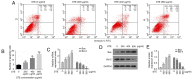
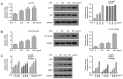
The consumption of smokeless tobacco extract (STE) is growing rapidly, and it has been implicated in several human diseases including diabetes, inflammation and a number of types of cancer. The toxicity of STE requires evaluation, as it is known to induce numerous public health issues. To investigate whether STE serves a role in cultured human oral mucosa fibroblasts (hOMFs), the present study examined HOMF morphology with inverted microscopy and immunofluorescence staining. The cell viability was measured with MTT assays, which detected the cell apoptosis rate via flow cytometry. The activities of reactive oxygen species (ROS), malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT) were measured via flow cytometry and commercial kits, subsequent to exposing the cells to various concentrations of STE. Reverse transcription quantitative polymerase chain reaction and western blot analyses were used to demonstrate that the mRNA and the protein expression levels of cell cycle-associated genes (cyclin-dependent kinase inhibitor 1 and cyclin D1), apoptosis-associated genes [B cell lymphoma 2 (Bcl-2) and Bcl-2-associatied X protein], tumor protein (p53), nuclear factor kappa light chain enhancer of activated B cells (NF-κB)-transcription factor (p65) signaling pathways, NF-E2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1) and NAD(P)H: quinoneoxidoreductase1 (NQO1). The results indicated that the hOMF cells were positive for cytokeratin staining. STE induced G1-S cell cycle progression and cell apoptosis by regulating the cell cycle or apoptosis-associated proteins. STE treatment increased the concentrations of ROS and MDA, and decreased the concentrations of SOD and CAT. STE unregulated phosphorylated-p53, NF-κB p65, Nrf2, HO-1, and NQO1 expression levels in the hOMF cells. The present study demonstrated that STE appears to promote oral disease.
Keywords: apoptosis; human oral mucosa fibroblast cells; nuclear factor kappa light chain enhancer of activated B cells; proliferation; smokeless tobacco extract; tumor protein 53.
Figures


Similar articles
-
Protective effects of antioxidants against smokeless tobacco-induced oxidative stress and modulation of Bcl-2 and p53 genes in human oral keratinocytes.Free Radic Res. 2001 Aug;35(2):181-94. doi: 10.1080/10715760100300731a. Free Radic Res. 2001. PMID: 11697199
-
Antioxidant tert-butylhydroquinone ameliorates arsenic-induced intracellular damages and apoptosis through induction of Nrf2-dependent antioxidant responses as well as stabilization of anti-apoptotic factor Bcl-2 in human keratinocytes.Free Radic Biol Med. 2016 May;94:74-87. doi: 10.1016/j.freeradbiomed.2016.02.009. Epub 2016 Feb 12. Free Radic Biol Med. 2016. PMID: 26878773
-
Pogostone attenuates TNF-α-induced injury in A549 cells via inhibiting NF-κB and activating Nrf2 pathways.Int Immunopharmacol. 2018 Sep;62:15-22. doi: 10.1016/j.intimp.2018.06.029. Epub 2018 Jun 30. Int Immunopharmacol. 2018. PMID: 29966943
-
Xanthoangelol Prevents Ox-LDL-Induced Endothelial Cell Injury by Activating Nrf2/ARE Signaling.J Cardiovasc Pharmacol. 2019 Aug;74(2):162-171. doi: 10.1097/FJC.0000000000000699. J Cardiovasc Pharmacol. 2019. PMID: 31356547
-
Garcinol, an acetyltransferase inhibitor, suppresses proliferation of breast cancer cell line MCF-7 promoted by 17β-estradiol.Asian Pac J Cancer Prev. 2014;15(12):5001-7. doi: 10.7314/apjcp.2014.15.12.5001. Asian Pac J Cancer Prev. 2014. PMID: 24998578
Cited by
-
Quantification of Nicotine and Cotinine in Plasma, Saliva, and Urine by HPLC Method in Chewing Tobacco Users.Asian Pac J Cancer Prev. 2019 Dec 1;20(12):3617-3623. doi: 10.31557/APJCP.2019.20.12.3617. Asian Pac J Cancer Prev. 2019. PMID: 31870102 Free PMC article.
-
Nicotine exacerbates liver damage in a mice model of Ehrlich ascites carcinoma through shifting SOD/NF-κB/caspase-3 pathways: ameliorating role of Chlorella vulgaris.Naunyn Schmiedebergs Arch Pharmacol. 2024 Oct;397(10):7767-7783. doi: 10.1007/s00210-024-03120-9. Epub 2024 May 9. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38722343 Free PMC article.
-
Toxic effects of smokeless tobacco on female reproductive health: A review.Curr Res Toxicol. 2022 Mar 7;3:100066. doi: 10.1016/j.crtox.2022.100066. eCollection 2022. Curr Res Toxicol. 2022. PMID: 35310558 Free PMC article. Review.
-
Aqueous extract of tobacco induces mitochondrial potential dependent cell death and epithelial-mesenchymal transition in gingival epithelial cells.Saudi J Biol Sci. 2021 Aug;28(8):4613-4618. doi: 10.1016/j.sjbs.2021.04.068. Epub 2021 May 1. Saudi J Biol Sci. 2021. PMID: 34354447 Free PMC article.
-
Salivary Oxidative Stress and Antioxidant Capacity in Smokeless Tobacco (Naswar) Users.Clin Cosmet Investig Dent. 2023 Jul 12;15:121-132. doi: 10.2147/CCIDE.S415827. eCollection 2023. Clin Cosmet Investig Dent. 2023. PMID: 37465099 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
