Dipeptide γ-secretase inhibitor treatment enhances the anti-tumor effects of cisplatin against gastric cancer by suppressing cancer stem cell properties
- PMID: 30250614
- PMCID: PMC6144782
- DOI: 10.3892/ol.2018.9301
Dipeptide γ-secretase inhibitor treatment enhances the anti-tumor effects of cisplatin against gastric cancer by suppressing cancer stem cell properties
Abstract
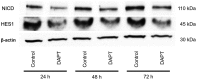
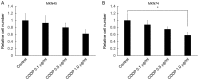
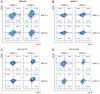
The γ-secretase inhibitor blocks Notch activity by preventing its cleavage at the cell surface. In the present study, the effect of the γ-secretase inhibitor on the viability of gastric cancer cells when administered in combination with cisplatin was investigated, with particular focus on CD44highLgr-5high cancer cells. The four gastric cancer cell lines, MKN45, MKN74, SC-6-JCK and SH-10-TC, were used for the experiments. In the MTT assay, treatment with 25 µM dipeptide γ-secretase inhibitor (DAPT) alone did not affect cell proliferation in any of the four cell lines. Gastric cancer cells subjected to combination treatment with DAPT and cisplatin exhibited decreased viability when compared with those treated with cisplatin alone. Flow cytometry was performed to evaluate the expression of cluster of differentiation (CD)-44 and leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr-5), two cancer stem cell markers in gastric cancers. Treatment with cisplatin alone significantly increased the proportion of CD44highLgr-5high cells. However, the addition of DAPT to cisplatin reduced the CD44highLgr-5high fraction, suggesting that DAPT reduced the number of gastric cancer cells. In conclusion, the present study demonstrated the synergistic effects of DAPT in combination with cisplatin by decreasing the survival of gastric cancer cells. In addition, combination treatment with DAPT reduced the number of CD44highLgr-5high cells, which are thought to exhibit cancer stem cell properties. These results highlight the therapeutic potential of DAPT in gastric cancer treatment.
Keywords: Notch pathway; cancer stem cell; gastric cancer; γ-secretase inhibitor.
Figures
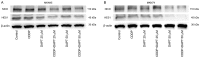


Similar articles
-
Gastric tumor-initiating CD44+ cells and epithelial-mesenchymal transition are inhibited by γ-secretase inhibitor DAPT.Oncol Lett. 2015 Nov;10(5):3293-3299. doi: 10.3892/ol.2015.3727. Epub 2015 Sep 18. Oncol Lett. 2015. PMID: 26722328 Free PMC article.
-
Combination treatment of all-trans retinoic acid (ATRA) and γ-secretase inhibitor (DAPT) cause growth inhibition and apoptosis induction in the human gastric cancer cell line.Cytotechnology. 2018 Apr;70(2):865-877. doi: 10.1007/s10616-018-0199-3. Epub 2018 Feb 7. Cytotechnology. 2018. PMID: 29417442 Free PMC article.
-
Pretreatment with the γ-secretase inhibitor DAPT sensitizes drug-resistant ovarian cancer cells to cisplatin by downregulation of Notch signaling.Int J Oncol. 2014 Apr;44(4):1401-9. doi: 10.3892/ijo.2014.2301. Epub 2014 Feb 14. Int J Oncol. 2014. PMID: 24535252
-
Notch pathway inhibition using DAPT, a γ-secretase inhibitor (GSI), enhances the antitumor effect of cisplatin in resistant osteosarcoma.Mol Carcinog. 2019 Jan;58(1):3-18. doi: 10.1002/mc.22873. Epub 2018 Nov 5. Mol Carcinog. 2019. PMID: 29964327
-
Concurrent Treatment with Anti-DLL4 Enhances Antitumor and Proapoptotic Efficacy of a γ-Secretase Inhibitor in Gastric Cancer.Transl Oncol. 2018 Jun;11(3):599-608. doi: 10.1016/j.tranon.2018.02.016. Epub 2018 Mar 16. Transl Oncol. 2018. PMID: 29571073 Free PMC article.
Cited by
-
Targeting Notch to Maximize Chemotherapeutic Benefits: Rationale, Advanced Strategies, and Future Perspectives.Cancers (Basel). 2021 Oct 12;13(20):5106. doi: 10.3390/cancers13205106. Cancers (Basel). 2021. PMID: 34680255 Free PMC article. Review.
-
LncRNA ADAMTS9-AS2 inhibits gastric cancer (GC) development and sensitizes chemoresistant GC cells to cisplatin by regulating miR-223-3p/NLRP3 axis.Aging (Albany NY). 2020 Jun 9;12(11):11025-11041. doi: 10.18632/aging.103314. Epub 2020 Jun 9. Aging (Albany NY). 2020. PMID: 32516127 Free PMC article.
-
Unlocking the Secrets of Cancer Stem Cells with γ-Secretase Inhibitors: A Novel Anticancer Strategy.Molecules. 2021 Feb 12;26(4):972. doi: 10.3390/molecules26040972. Molecules. 2021. PMID: 33673088 Free PMC article. Review.
-
Laminin-411 and -511 Modulate the Proliferation, Adhesion, and Morphology of Gastric Cancer Cells.Cell Biochem Biophys. 2021 Jun;79(2):407-418. doi: 10.1007/s12013-021-00972-3. Epub 2021 Feb 25. Cell Biochem Biophys. 2021. PMID: 33629255
References
-
- Cancer Information Service, National Cancer Center, corp-author. http://ganjoho.jp/en/professional/statistics/table_download.html Cancer mortality from Vital Statistics in Japan (1958–2016)
-
- Dawood S, Austin L, Cristofanilli M. Cancer stem cells: Implications for cancer therapy. Oncology (Williston Park) 2014;28:1101–1107, 1110. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
