CHIIMP: An automated high-throughput microsatellite genotyping platform reveals greater allelic diversity in wild chimpanzees
- PMID: 30250675
- PMCID: PMC6145012
- DOI: 10.1002/ece3.4302
CHIIMP: An automated high-throughput microsatellite genotyping platform reveals greater allelic diversity in wild chimpanzees
Abstract
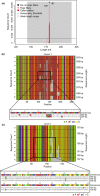
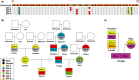
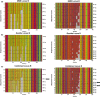
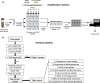
Short tandem repeats (STRs), also known as microsatellites, are commonly used to noninvasively genotype wild-living endangered species, including African apes. Until recently, capillary electrophoresis has been the method of choice to determine the length of polymorphic STR loci. However, this technique is labor intensive, difficult to compare across platforms, and notoriously imprecise. Here we developed a MiSeq-based approach and tested its performance using previously genotyped fecal samples from long-term studied chimpanzees in Gombe National Park, Tanzania. Using data from eight microsatellite loci as a reference, we designed a bioinformatics platform that converts raw MiSeq reads into locus-specific files and automatically calls alleles after filtering stutter sequences and other PCR artifacts. Applying this method to the entire Gombe population, we confirmed previously reported genotypes, but also identified 31 new alleles that had been missed due to sequence differences and size homoplasy. The new genotypes, which increased the allelic diversity and heterozygosity in Gombe by 61% and 8%, respectively, were validated by replicate amplification and pedigree analyses. This demonstrated inheritance and resolved one case of an ambiguous paternity. Using both singleplex and multiplex locus amplification, we also genotyped fecal samples from chimpanzees in the Greater Mahale Ecosystem in Tanzania, demonstrating the utility of the MiSeq-based approach for genotyping nonhabituated populations and performing comparative analyses across field sites. The new automated high-throughput analysis platform (available at https://github.com/ShawHahnLab/chiimp) will allow biologists to more accurately and effectively determine wildlife population size and structure, and thus obtain information critical for conservation efforts.
Keywords: Pan troglodytes; high‐throughput STR genotyping; length homoplasy; parentage analysis; short tandem repeats.
Figures

References
-
- Adams, R. I. , Brown, K. M. , & Hamilton, M. B. (2004). The impact of microsatellite electromorph size homoplasy on multilocus population structure estimates in a tropical tree (Corythophora alta) and an anadromous fish (Morone saxatilis). Molecular Ecology, 13, 2579–2588. 10.1111/j.1365-294X.2004.02256.x - DOI - PubMed
-
- Arandjelovic, M. , Guschanski, K. , Schubert, G. , Harris, T. R. , Thalmann, O. , Siedel, H. , & Vigilant, L. (2009). Two‐step multiplex polymerase chain reaction improves the speed and accuracy of genotyping using DNA from noninvasive and museum samples. Molecular Ecology Resources, 9, 28–36. 10.1111/j.1755-0998.2008.02387.x - DOI - PubMed
-
- Barbian, H. J. , Li, Y. , Ramirez, M. , Klase, Z. , Lipende, I. , Mjungu, D. , … Hahn, B. H. (2018). Destabilization of the gut microbiome marks the end‐stage of simian immunodeficiency virus infection in wild chimpanzees. American Journal of Primatology, 80, e22515 10.1002/ajp.22515 - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

