Laboulbeniales hyperparasites (Fungi, Ascomycota) of bat flies: Independent origins and host associations
- PMID: 30250711
- PMCID: PMC6145224
- DOI: 10.1002/ece3.4359
Laboulbeniales hyperparasites (Fungi, Ascomycota) of bat flies: Independent origins and host associations
Abstract
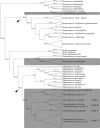
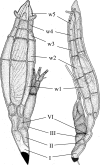
The aim of this study was to explore the diversity of ectoparasitic fungi (Ascomycota, Laboulbeniales) that use bat flies (Diptera, Hippoboscoidea) as hosts. Bat flies themselves live as ectoparasites on the fur and wing membranes of bats (Mammalia, Chiroptera); hence this is a tripartite parasite system. Here, we collected bats, bat flies, and Laboulbeniales, and conducted phylogenetic analyses of Laboulbeniales to contrast morphology with ribosomal sequence data. Parasitism of bat flies by Laboulbeniales arose at least three times independently, once in the Eastern Hemisphere (Arthrorhynchus) and twice in the Western Hemisphere (Gloeandromyces, Nycteromyces). We hypothesize that the genera Arthrorhynchus and Nycteromyces evolved independently from lineages of ectoparasites of true bugs (Hemiptera). We assessed phylogenetic diversity of the genus Gloeandromyces by considering the LSU rDNA region. Phenotypic plasticity and position-induced morphological adaptations go hand in hand. Different morphotypes belong to the same phylogenetic species. Two species, G. pageanus and G. streblae, show divergence by host utilization. In our assessment of coevolution, we only observe congruence between the Old World clades of bat flies and Laboulbeniales. The other associations are the result of the roosting ecology of the bat hosts. This study has considerably increased our knowledge about bats and their associated ectoparasites and shown the necessity of including molecular data in Laboulbeniales taxonomy.
Keywords: Ascomycota; ectoparasites; host specialization; phenotypic plasticity; ribosomal DNA; taxonomy.
Figures






Similar articles
-
A tripartite survey of hyperparasitic fungi associated with ectoparasitic flies on bats (Mammalia: Chiroptera) in a neotropical cloud forest in Panama.Parasite. 2018;25:19. doi: 10.1051/parasite/2018017. Epub 2018 Apr 10. Parasite. 2018. PMID: 29633707 Free PMC article.
-
Laboulbeniales (Fungi: Ascomycota) infection of bat flies (Diptera: Nycteribiidae) from Miniopterus schreibersii across Europe.Parasit Vectors. 2018 Jul 5;11(1):395. doi: 10.1186/s13071-018-2921-6. Parasit Vectors. 2018. PMID: 29976258 Free PMC article.
-
Parasites of parasites of bats: Laboulbeniales (Fungi: Ascomycota) on bat flies (Diptera: Nycteribiidae) in central Europe.Parasit Vectors. 2017 Feb 21;10(1):96. doi: 10.1186/s13071-017-2022-y. Parasit Vectors. 2017. PMID: 28222795 Free PMC article.
-
Bats, Bat Flies, and Fungi: A Case of Hyperparasitism.Trends Parasitol. 2018 Sep;34(9):784-799. doi: 10.1016/j.pt.2018.06.006. Trends Parasitol. 2018. PMID: 30097262 Review.
-
Against all odds: explaining high host specificity in dispersal-prone parasites.Int J Parasitol. 2007 Jul;37(8-9):871-6. doi: 10.1016/j.ijpara.2007.02.004. Epub 2007 Feb 20. Int J Parasitol. 2007. PMID: 17382332 Review.
Cited by
-
New insights into the DNA extraction and PCR amplification of minute ascomycetes in the genus Laboulbenia (Pezizomycotina, Laboulbeniales).IMA Fungus. 2024 Jun 11;15(1):14. doi: 10.1186/s43008-024-00146-9. IMA Fungus. 2024. PMID: 38863065 Free PMC article.
-
Hyperparasitic Fungi on Black Mildews (Meliolales, Ascomycota): Hidden Fungal Diversity in the Tropics.Front Fungal Biol. 2022 May 24;3:885279. doi: 10.3389/ffunb.2022.885279. eCollection 2022. Front Fungal Biol. 2022. PMID: 37746226 Free PMC article. Review.
-
Hyperparasitism in bat flies (Diptera: Streblidae): new records and interaction networks in the Neotropics.Parasitol Res. 2024 Jun 26;123(6):255. doi: 10.1007/s00436-024-08221-1. Parasitol Res. 2024. PMID: 38922514 Free PMC article.
-
The paradoxical rarity of a fruit fly fungus attacking a broad range of hosts.Ecol Evol. 2020 Jul 20;10(16):8871-8879. doi: 10.1002/ece3.6585. eCollection 2020 Aug. Ecol Evol. 2020. PMID: 32884663 Free PMC article.
-
Morphological species of Gloeandromyces (Ascomycota, Laboulbeniales) evaluated using single-locus species delimitation methods.Fungal Syst Evol. 2019 Jun;3:19-33. doi: 10.3114/fuse.2019.03.03. Epub 2019 Jan 11. Fungal Syst Evol. 2019. PMID: 32467897 Free PMC article.
References
-
- Barquez, R. M. , Perez, S. , Miller, B. , & Diaz, M. (2015). Artibeus lituratus. The IUCN red list of threatened species 2015. Available at http://www.iucnredlist.org/details/2136/0 (accessed 06 October 2017).
-
- Benjamin, R. K. (1971). Introduction and supplement to Roland Thaxter's contribution towards a monograph of the Laboulbeniaceae. Bibliotheca Mycologica, 30, 1–155.
-
- Benjamin, R. K. (1981). Laboulbeniales on semiaquatic Hemiptera. IV. Addenda to Prolixandromyces. Aliso, 10, 1–17. 10.5642/aliso - DOI
-
- Benjamin, R. K. (1992a). Cupulomyces, a new genus of Laboulbeniales (Ascomycetes) based on Stigmatomyces lasiochili . Aliso, 13, 355–364. 10.5642/aliso - DOI
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

