Identification and characterization of in vivo, in vitro and reactive metabolites of vandetanib using LC-ESI-MS/MS
- PMID: 30251155
- PMCID: PMC6768145
- DOI: 10.1186/s13065-018-0467-5
Identification and characterization of in vivo, in vitro and reactive metabolites of vandetanib using LC-ESI-MS/MS
Abstract
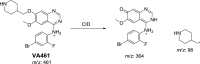
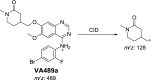
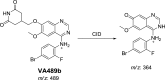
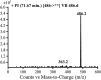
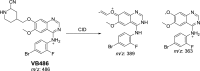
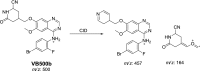
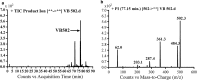
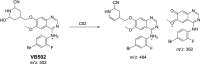
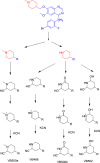
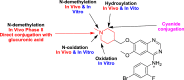
Vandetanib (Caprelsa tablets, VNT) is an orally inhibitor of vascular endothelial growth factor receptor 2. The current research reports the characterization and identification of in vitro, in vivo and reactive intermediates of VNT. In vitro metabolites of VNT were performed by incubation with rat liver microsomes (RLMs). Extraction of vandetanib and its in vitro metabolites from the incubation mixtures were done by protein precipitation. In vivo metabolism was done by giving one oral dose of vandetanib (30.8 mg/kg) to Sprague Dawley rats in metabolic cages by using oral gavage. Urine was gathered then filtered at certain time intervals (0, 6, 12, 18, 24, 48, 72, 96 and 120 h) from vandetanib dosing. A similar volume of ACN was added to each collected urine sample. Both layers (organic and aqueous) were injected into liquid chromatography electro spray ionization tandem mass spectrometry (LC-ESI-MS/MS) to detect in vivo vandetanib metabolites. N-methyl piperidine ring of vandetanib is considered a cyclic tertiary amine that undergoes metabolism forming iminium intermediates that are very reactive toward nucleophilic macromolecules. Incubation of vandetanib with RLMs in the presence of 1.0 mM KCN was made to check reactive metabolites as it is usually responsible for noticeable idiosyncratic toxicities including phototoxicity and QT interval prolongation. Four in vivo phase I, one in vivo phase II metabolites, six in vitro phase I metabolites and four cyano conjugates of vandetanib were detected by LC-MS/MS. In vitro and in vivo phase I metabolic reactions were N-oxide formation, N-demethylation, α-carbonyl formation and α-hydroxylation. In vivo phase II metabolic reaction was direct conjugation of vandetanib with glucuronic acid. All metabolic reactions occurred in N-methyl piperidine of vandetanib which causes toxicity and instability of vandetanib.
Keywords: Cyano conjugates; In vitro metabolites; In vivo metabolites; N-methyl piperidine; Vandetanib.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






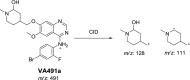




Similar articles
-
Identification and characterization of in silico, in vivo, in vitro, and reactive metabolites of infigratinib using LC-ITMS: bioactivation pathway elucidation and in silico toxicity studies of its metabolites.RSC Adv. 2020 Apr 23;10(28):16231-16244. doi: 10.1039/c9ra10871h. eCollection 2020 Apr 23. RSC Adv. 2020. PMID: 35498820 Free PMC article.
-
Profiling of in vivo, in vitro and reactive zorifertinib metabolites using liquid chromatography ion trap mass spectrometry.RSC Adv. 2022 Jul 21;12(32):20991-21003. doi: 10.1039/d2ra02848d. eCollection 2022 Jul 14. RSC Adv. 2022. PMID: 35919181 Free PMC article.
-
Identification and characterization of in vitro, in vivo, and reactive metabolites of tandutinib using liquid chromatography ion trap mass spectrometry.Anal Methods. 2021 Jan 28;13(3):399-410. doi: 10.1039/d0ay02106g. Anal Methods. 2021. PMID: 33410830
-
Characterization of in vivo metabolites in rat urine following an oral dose of masitinib by liquid chromatography tandem mass spectrometry.Chem Cent J. 2018 May 15;12(1):61. doi: 10.1186/s13065-018-0429-y. Chem Cent J. 2018. PMID: 29766296 Free PMC article.
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
Cited by
-
Identification and characterization of in silico, in vivo, in vitro, and reactive metabolites of infigratinib using LC-ITMS: bioactivation pathway elucidation and in silico toxicity studies of its metabolites.RSC Adv. 2020 Apr 23;10(28):16231-16244. doi: 10.1039/c9ra10871h. eCollection 2020 Apr 23. RSC Adv. 2020. PMID: 35498820 Free PMC article.
-
Liquid chromatography-tandem mass spectrometry metabolic profiling of nazartinib reveals the formation of unexpected reactive metabolites.R Soc Open Sci. 2019 Aug 14;6(8):190852. doi: 10.1098/rsos.190852. eCollection 2019 Aug. R Soc Open Sci. 2019. PMID: 31598253 Free PMC article.
-
Profiling of in vivo, in vitro and reactive zorifertinib metabolites using liquid chromatography ion trap mass spectrometry.RSC Adv. 2022 Jul 21;12(32):20991-21003. doi: 10.1039/d2ra02848d. eCollection 2022 Jul 14. RSC Adv. 2022. PMID: 35919181 Free PMC article.
-
Identification of reactive intermediate formation and bioactivation pathways in Abemaciclib metabolism by LC-MS/MS: in vitro metabolic investigation.R Soc Open Sci. 2019 Jan 23;6(1):181714. doi: 10.1098/rsos.181714. eCollection 2019 Jan. R Soc Open Sci. 2019. PMID: 30800400 Free PMC article.
-
Pharmacokinetics, Bioavailability, Excretion and Metabolism Studies of Akebia Saponin D in Rats: Causes of the Ultra-Low Oral Bioavailability and Metabolic Pathway.Front Pharmacol. 2021 Apr 15;12:621003. doi: 10.3389/fphar.2021.621003. eCollection 2021. Front Pharmacol. 2021. PMID: 33935711 Free PMC article.
References
-
- Thornton K, Kim G, Maher VE, Chattopadhyay S, Tang S, Moon YJ, et al. Vandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease: U.S. Food and Drug Administration drug approval summary. Clin Cancer Res. 2012;18(14):3722–3730. doi: 10.1158/1078-0432.CCR-12-0411. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

