Lymphocytes are a major source of circulating soluble dipeptidyl peptidase 4
- PMID: 30251416
- PMCID: PMC6194339
- DOI: 10.1111/cei.13163
Lymphocytes are a major source of circulating soluble dipeptidyl peptidase 4
Abstract
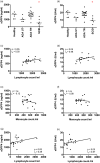
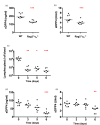
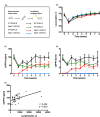
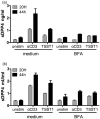
Dipeptidyl peptidase 4 (DPP4, CD26) is a serine protease that is expressed constitutively by many haematopoietic and non-haematopoietic tissues. It exists as a membrane-associated protein, as well as in an active, soluble form (herein called sDPP4), present at high concentrations in bodily fluids. Despite the proposed use of sDPP4 as a biomarker for multiple diseases, its cellular sources are not well defined. Here, we report that individuals with congenital lymphocyte immunodeficiency had markedly lower serum concentrations of sDPP4, which were restored upon successful treatment and restoration of lymphocyte haematopoiesis. Using irradiated lymphopenic mice and wild-type to Dpp4-/- reciprocal bone marrow chimeric animals, we found that haematopoietic cells were a major source of circulating sDPP4. Furthermore, activation of human and mouse T lymphocytes resulted in increased sDPP4, providing a mechanistic link between immune system activation and sDPP4 concentration. Finally, we observed that acute viral infection induced a transient increase in sDPP4, which correlated with the expansion of antigen-specific CD8+ T cell responses. Our study demonstrates that sDPP4 concentrations are determined by the frequency and activation state of lymphocyte populations. Insights from these studies will support the use of sDPP4 concentration as a biomarker for inflammatory and infectious diseases.
Keywords: T cells; chemokines; immunodeficiency diseases.
© 2018 British Society for Immunology.
Figures
References
-
- McCaughan GW, Wickson JE, Creswick PF, Gorrell MD. Identification of the bile canalicular cell‐surface molecule GP110 as the ectopeptidase dipeptidyl peptidase‐IV – an analysis by tissue distribution, purification and N‐terminal amino‐acid‐sequence. Hepatology 1990; 11:534–44. - PubMed
-
- Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science 1993; 261:466–9. - PubMed
-
- Chien CH, Huang LH, Chou CY et al One site mutation disrupts dimer formation in human DPP‐IV proteins. J Biol Chem 2004; 279:52338–45. - PubMed
-
- Durinx C, Lambeir AM, Bosmans E et al Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is responsible for the release of X‐Pro dipeptides. Eur J Biochem 2000; 267:5608–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

