Role of Cardiac Magnetic Resonance Imaging in Patients with Idiopathic Ventricular Arrhythmias
- PMID: 30251607
- PMCID: PMC6367696
- DOI: 10.2174/1573403X14666180925095923
Role of Cardiac Magnetic Resonance Imaging in Patients with Idiopathic Ventricular Arrhythmias
Abstract
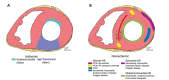
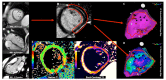
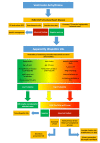
Ventricular Arrhythmias (VAs) may present with a wide spectrum of clinical manifestations ranging from mildly symptomatic frequent premature ventricular contractions to lifethreatening events such as sustained ventricular tachycardia, ventricular fibrillation and sudden cardiac death. Myocardial scar plays a central role in the genesis and maintenance of re-entrant arrhythmias which are commonly associated with Structural Heart Diseases (SHD) such as ischemic heart disease, healed myocarditis and non-ischemic cardiomyopathies. However, the arrhythmogenic substrate may remain unclear in up to 50% of the cases after a routine diagnostic workup, comprehensive of 12-lead surface ECG, transthoracic echocardiography and coronary angiography/ computed tomography. Whenever any abnormality cannot be identified, VAs are referred as to "idiopathic". In the last decade, Cardiac Magnetic Resonance (CMR) imaging has acquired a growing role in the identification and characterization of myocardial arrhythmogenic substrate, not only being able to accurately and reproducibly quantify biventricular function, but, more importantly, providing information about the presence of myocardial structural abnormalities such as myocardial fatty replacement, myocardial oedema, and necrosis/ fibrosis, which may otherwise remain unrecognized. Moreover, CMR has recently demonstrated to be of great value in guiding interventional treatments, such as radiofrequency ablation, by reliably identifying VA sites of origin and improving long-term outcomes. In the present manuscript, we review the available data regarding the utility of CMR in the workup of apparently "idiopathic" VAs with a special focus on its prognostic relevance and its application in planning and guiding interventional treatments.
Keywords: Cardiac magnetic resonance; idiopathic; late gadolinium enhancement; structural heart disease; tissue characterization; ventricular arrhythmias..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures





References
-
- Tan A.Y., Ellenbogen K. Ventricular arrhythmias in apparently normal hearts. Card. Electrophysiol. Clin. 2016;8:613–621. - PubMed
-
- Nucifora G., Muser D., Masci P.G., et al. Prevalence and prognostic value of concealed structural abnormalities in patients with apparently idiopathic ventricular arrhythmias of left versus right ventricular origin A magnetic resonance imaging study. Circ Arrhythm Electrophysiol. 2014;7:456–462. - PubMed
-
- Mahida S., Sacher F., Dubois R., et al. Cardiac imaging in patients with ventricular tachycardia. Circulation. 2017;136:2491–2507. - PubMed
-
- Latif S., Dixit S., Callans D.J. Ventricular arrhythmias in normal hearts. Cardiol. Clin. 2008;26:367–380. - PubMed
-
- Saksena S., Camm A.J. Electrophysiological disorders of the heart: Expert consult. Elsevier Health Sciences; 2011.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

