Lysine as a heme iron ligand: A property common to three truncated hemoglobins from Chlamydomonas reinhardtii
- PMID: 30251657
- PMCID: PMC6214630
- DOI: 10.1016/j.bbagen.2018.08.009
Lysine as a heme iron ligand: A property common to three truncated hemoglobins from Chlamydomonas reinhardtii
Abstract
Background: The nuclear genome of Chlamydomonas reinhardtii encodes a dozen hemoglobins of the truncated lineage. Four of these, named THB1-4, contain a single ~130-residue globin unit. THB1, which is cytoplasmic and capable of nitric oxide dioxygenation activity, uses a histidine and a lysine as axial ligands to the heme iron. In the present report, we compared THB2, THB3, and THB4 to THB1 to gain structural and functional insights into algal globins.
Methods: We inspected properties of the globin domains prepared by recombinant means through site-directed mutagenesis, electronic absorption, CD, and NMR spectroscopies, and X-ray crystallography.
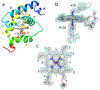
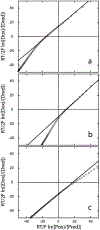
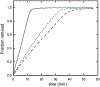
Results: Recombinant THB3, which lacks the proximal histidine but has a distal histidine, binds heme weakly. NMR data demonstrate that the recombinant domains of THB2 and THB4 coordinate the ferrous heme iron with the proximal histidine and a lysine from the distal helix. An X-ray structure of ferric THB4 confirms lysine coordination. THB1, THB2, and THB4 have reduction potentials between -65 and -100 mV, are capable of nitric oxide dioxygenation, are reduced at different rates by the diaphorase domain of C. reinhardtii nitrate reductase, and show different response to peroxide treatment.
Conclusions: Three single-domain C. reinhardtii hemoglobins use lysine as a distal heme ligand in both Fe(III) and Fe(II) oxidation states. This common feature is likely related to enzymatic activity in the management of reactive oxygen species.
General significance: Primary structure analysis of hemoglobins has limited power in the prediction of heme ligation. Experimental determination reveals variations in this essential property across the superfamily.
Keywords: 2-over-2 hemoglobin; Heme reduction potential; Nitric oxide dioxygenase; Nuclear magnetic resonance.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures

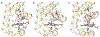

Similar articles
-
Dynamics of Lysine as a Heme Axial Ligand: NMR Analysis of the Chlamydomonas reinhardtii Hemoglobin THB1.Biochemistry. 2017 Jan 31;56(4):551-569. doi: 10.1021/acs.biochem.6b00926. Epub 2017 Jan 18. Biochemistry. 2017. PMID: 28032976
-
Structure of Chlamydomonas reinhardtii THB1, a group 1 truncated hemoglobin with a rare histidine-lysine heme ligation.Acta Crystallogr F Struct Biol Commun. 2015 Jun;71(Pt 6):718-25. doi: 10.1107/S2053230X15006949. Epub 2015 May 20. Acta Crystallogr F Struct Biol Commun. 2015. PMID: 26057801 Free PMC article.
-
Characterization of THB1, a Chlamydomonas reinhardtii truncated hemoglobin: linkage to nitrogen metabolism and identification of lysine as the distal heme ligand.Biochemistry. 2014 Jul 22;53(28):4573-89. doi: 10.1021/bi5005206. Epub 2014 Jul 9. Biochemistry. 2014. PMID: 24964018 Free PMC article.
-
Truncated hemoglobins and nitric oxide action.IUBMB Life. 2003 Oct-Nov;55(10-11):623-7. doi: 10.1080/15216540310001628708. IUBMB Life. 2003. PMID: 14711009 Review.
-
Structural bases for heme binding and diatomic ligand recognition in truncated hemoglobins.J Inorg Biochem. 2005 Jan;99(1):97-109. doi: 10.1016/j.jinorgbio.2004.10.035. J Inorg Biochem. 2005. PMID: 15598494 Review.
Cited by
-
Bacterial hemophilin homologs and their specific type eleven secretor proteins have conserved roles in heme capture and are diversifying as a family.J Bacteriol. 2024 Jun 20;206(6):e0044423. doi: 10.1128/jb.00444-23. Epub 2024 Mar 20. J Bacteriol. 2024. PMID: 38506530 Free PMC article.
-
BindWeb: A web server for ligand binding residue and pocket prediction from protein structures.Protein Sci. 2022 Dec;31(12):e4462. doi: 10.1002/pro.4462. Protein Sci. 2022. PMID: 36190332 Free PMC article.
-
A Heme Propionate Staples the Structure of Cytochrome c for Methionine Ligation to the Heme Iron.Inorg Chem. 2019 Oct 21;58(20):14085-14106. doi: 10.1021/acs.inorgchem.9b02111. Epub 2019 Oct 7. Inorg Chem. 2019. PMID: 31589413 Free PMC article.
-
Replacement of the heme axial lysine as a test of conformational adaptability in the truncated hemoglobin THB1.J Inorg Biochem. 2019 Dec;201:110824. doi: 10.1016/j.jinorgbio.2019.110824. Epub 2019 Sep 4. J Inorg Biochem. 2019. PMID: 31514090 Free PMC article.
-
A new iron supplement: The chelate of pig skin collagen peptide and Fe2+ can treat iron-deficiency anemia by modulating intestinal flora.Front Nutr. 2022 Dec 22;9:1055725. doi: 10.3389/fnut.2022.1055725. eCollection 2022. Front Nutr. 2022. PMID: 36618683 Free PMC article.
References
-
- Johnson EA, Rice SL, Preimesberger MR, Nye DB, Gilevicius L, Wenke BB, Brown JM, Witman GB, Lecomte JTJ, Characterization of THB1, a Chlamydomonas reinhardtii truncated hemoglobin: linkage to nitrogen metabolism and identification of lysine as the distal heme ligand, Biochemistry, 53 (2014) 4573–4589. doi:10.1021/bi5005206 - DOI - PMC - PubMed
-
- Rice SL, Boucher LE, Schlessman JL, Preimesberger MR, Bosch J, Lecomte JTJ, Structure of Chlamydomonas reinhardtii THB1, a group 1 truncated hemoglobin with a rare histidine-lysine heme ligation, Acta Crystallogr. F Struct. Biol. Commun, 71 (2015) 718–725. doi:10.1107/S2053230X15006949 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

