Reactive cysteine residues in the oxidative dimerization and Cu2+ induced aggregation of human γD-crystallin: Implications for age-related cataract
- PMID: 30251679
- PMCID: PMC6590075
- DOI: 10.1016/j.bbadis.2018.08.021
Reactive cysteine residues in the oxidative dimerization and Cu2+ induced aggregation of human γD-crystallin: Implications for age-related cataract
Abstract
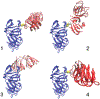
Cysteine (Cys) residues are major causes of crystallin disulfide formation and aggregation in aging and cataractous human lenses. We recently found that disulfide linkages are highly and partly conserved in β- and γ-crystallins, respectively, in human age-related nuclear cataract and glutathione depleted LEGSKO mouse lenses, and could be mimicked by in vitro oxidation. Here we determined which Cys residues are involved in disulfide-mediated crosslinking of recombinant human γD-crystallin (hγD). In vitro diamide oxidation revealed dimer formation by SDS-PAGE and LC-MS analysis with Cys 111-111 and C111-C19 as intermolecular disulfides and Cys 111-109 as intramolecular sites. Mutation of Cys111 to alanine completely abolished dimerization. Addition of αB-crystallin was unable to protect Cys 111 from dimerization. However, Cu2+-induced hγD-crystallin aggregation was suppressed up to 50% and 80% by mutants C109A and C111A, respectively, as well as by total glutathionylation. In contrast to our recently published results using ICAT-labeling method, manual mining of the same database confirmed the specific involvement of Cys111 in disulfides with no free Cys111 detectable in γD-crystallin from old and cataractous human lenses. Surface accessibility studies show that Cys111 in hγD is the most exposed Cys residue (29%), explaining thereby its high propensity toward oxidation and polymerization in the aging lens.
Keywords: Age related cataract; Copper oxidation; Cysteine disulfide; Human gamma D crystallin.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures

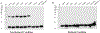


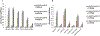


Similar articles
-
Evidence of Highly Conserved β-Crystallin Disulfidome that Can be Mimicked by In Vitro Oxidation in Age-related Human Cataract and Glutathione Depleted Mouse Lens.Mol Cell Proteomics. 2015 Dec;14(12):3211-23. doi: 10.1074/mcp.M115.050948. Epub 2015 Oct 9. Mol Cell Proteomics. 2015. PMID: 26453637 Free PMC article.
-
Dynamic disulfide exchange in a crystallin protein in the human eye lens promotes cataract-associated aggregation.J Biol Chem. 2018 Nov 16;293(46):17997-18009. doi: 10.1074/jbc.RA118.004551. Epub 2018 Sep 21. J Biol Chem. 2018. PMID: 30242128 Free PMC article.
-
The Structure and Stability of the Disulfide-Linked γS-Crystallin Dimer Provide Insight into Oxidation Products Associated with Lens Cataract Formation.J Mol Biol. 2019 Feb 1;431(3):483-497. doi: 10.1016/j.jmb.2018.12.005. Epub 2018 Dec 13. J Mol Biol. 2019. PMID: 30552875
-
The Functional Significance of High Cysteine Content in Eye Lens γ-Crystallins.Biomolecules. 2024 May 17;14(5):594. doi: 10.3390/biom14050594. Biomolecules. 2024. PMID: 38786000 Free PMC article. Review.
-
Redox chemistry of lens crystallins: A system of cysteines.Exp Eye Res. 2021 Oct;211:108707. doi: 10.1016/j.exer.2021.108707. Epub 2021 Jul 29. Exp Eye Res. 2021. PMID: 34332989 Free PMC article. Review.
Cited by
-
ATCUN-like Copper Site in βB2-Crystallin Plays a Protective Role in Cataract-Associated Aggregation.Inorg Chem. 2023 Jul 10;62(27):10592-10604. doi: 10.1021/acs.inorgchem.3c00794. Epub 2023 Jun 28. Inorg Chem. 2023. PMID: 37379524 Free PMC article.
-
A native chemical chaperone in the human eye lens.Elife. 2022 Jun 20;11:e76923. doi: 10.7554/eLife.76923. Elife. 2022. PMID: 35723573 Free PMC article.
-
Zinc and Copper Ions Induce Aggregation of Human β-Crystallins.Molecules. 2022 May 6;27(9):2970. doi: 10.3390/molecules27092970. Molecules. 2022. PMID: 35566320 Free PMC article.
-
Oxidation of active cysteines mediates protein aggregation of S10R, the cataract-associated mutant of mouse GammaB-crystallin.Proteins. 2022 Nov;90(11):1987-2000. doi: 10.1002/prot.26391. Epub 2022 Jul 7. Proteins. 2022. PMID: 35726360 Free PMC article.
-
The Eye Lens Protein, γS Crystallin, Undergoes Glutathionylation-Induced Disulfide Bonding Between Cysteines 22 and 26.Biomolecules. 2025 Mar 11;15(3):402. doi: 10.3390/biom15030402. Biomolecules. 2025. PMID: 40149938 Free PMC article.
References
-
- Jedziniak JA, Kinshita JH, Yates EM, Hocker LO, Benedek GB, On the presence and mechanism of formation of heavy molecular weight aggregates in human normal and cataractous lenses, Exp. Eye Res. 15 (1973) 185–192. - PubMed
-
- Truscott RJ, Augusteyn RC, Oxidative changes in human lens proteins during senile nuclear cataract formation, Biochim. Biophys. Acta 492 (1977) 43–52. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

