Role of p38γ MAPK in regulation of EMT and cancer stem cells
- PMID: 30251680
- PMCID: PMC6428452
- DOI: 10.1016/j.bbadis.2018.08.024
Role of p38γ MAPK in regulation of EMT and cancer stem cells
Abstract
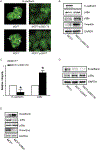
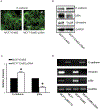
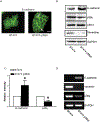
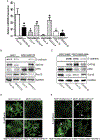
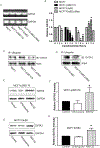
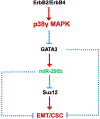
p38γ is a member of p38 MAPK family which contains four isoforms p38α, p38β, p38γ, and p38δ. p38γ MAPK has unique function and is less investigated. Recent studies revealed that p38γ MAPK may be involved in tumorigenesis and cancer aggressiveness. However, the underlying cellular/molecular mechanisms remain unclear. Epithelial-mesenchymal transition (EMT) is a process that epithelial cancer cells transform to facilitate the loss of epithelial features and gain of mesenchymal phenotype. EMT promotes cancer cell progression and metastasis, and is involved in the regulation of cancer stem cells (CSCs) which have self-renewal capacity and are resistant to chemotherapy and target therapy. We showed that p38γ MAPK significantly increased EMT in breast cancer cells; over-expression of p38γ MAPK enhanced EMT while its down-regulation inhibited EMT. Meanwhile, p38γ MAPK augmented CSC population while knock down of p38γ MAPK decreased CSC ratio in breast cancer cells. MicroRNA-200b (miR-200b) was down-stream of p38γ MAPK and inhibited by p38γ MAPK; miR-200b mimics blocked p38γ MAPK-induced EMT while miR-200b inhibitors promoted EMT. p38γ MAPK regulated miR-200b through inhibiting GATA3. p38γ MAPK induced GATA3 ubiquitination, leading to its proteasome-dependent degradation. Suz12, a Polycomb group protein, was down-stream of miR-200b and involved in miR-200b regulation of EMT. Thus, our study established an important role of p38γ MAPK in EMT and identified a novel signaling pathway for p38γ MAPK-mediated tumor promotion.
Keywords: Cancer stem cells; Metastasis; MicroRNA; p38γ MAPK.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors declare that they have no competing interests.
Figures


Similar articles
-
ErbB2 and p38γ MAPK mediate alcohol-induced increase in breast cancer stem cells and metastasis.Mol Cancer. 2016 Jul 14;15(1):52. doi: 10.1186/s12943-016-0532-4. Mol Cancer. 2016. PMID: 27416801 Free PMC article.
-
miR-200b regulates epithelial-mesenchymal transition of chemo-resistant breast cancer cells by targeting FN1.Discov Med. 2017 Sep;24(131):75-85. Discov Med. 2017. PMID: 28972876
-
Dual regulation by microRNA-200b-3p and microRNA-200b-5p in the inhibition of epithelial-to-mesenchymal transition in triple-negative breast cancer.Oncotarget. 2015 Jun 30;6(18):16638-52. doi: 10.18632/oncotarget.3184. Oncotarget. 2015. PMID: 26062653 Free PMC article.
-
Review of miR-200b and cancer chemosensitivity.Biomed Pharmacother. 2012 Sep;66(6):397-402. doi: 10.1016/j.biopha.2012.06.002. Epub 2012 Jun 29. Biomed Pharmacother. 2012. PMID: 22795796 Review.
-
P38 kinase in gastrointestinal cancers.Cancer Gene Ther. 2023 Sep;30(9):1181-1189. doi: 10.1038/s41417-023-00622-1. Epub 2023 May 29. Cancer Gene Ther. 2023. PMID: 37248432 Free PMC article. Review.
Cited by
-
Dual specificity phosphatase 22 suppresses mesangial cell hyperproliferation, fibrosis, inflammation and the MAPK signaling pathway in diabetic nephropathy.Exp Ther Med. 2022 Nov 7;24(6):744. doi: 10.3892/etm.2022.11680. eCollection 2022 Dec. Exp Ther Med. 2022. PMID: 36561966 Free PMC article.
-
Tobacco smoke induced hepatic cancer stem cell-like properties through IL-33/p38 pathway.J Exp Clin Cancer Res. 2019 Jan 28;38(1):39. doi: 10.1186/s13046-019-1052-z. J Exp Clin Cancer Res. 2019. PMID: 30691509 Free PMC article.
-
Transcriptomic analysis revealed potential regulatory biomarkers and repurposable drugs for breast cancer treatment.Cancer Rep (Hoboken). 2024 May;7(5):e2009. doi: 10.1002/cnr2.2009. Cancer Rep (Hoboken). 2024. PMID: 38717954 Free PMC article.
-
Follistatin Is a Novel Chemoattractant for Migration and Invasion of Placental Trophoblasts of Mice.Cells. 2022 Nov 29;11(23):3816. doi: 10.3390/cells11233816. Cells. 2022. PMID: 36497076 Free PMC article.
-
Role of p38 MAP kinase in cancer stem cells and metastasis.Oncogene. 2022 Jun;41(23):3177-3185. doi: 10.1038/s41388-022-02329-3. Epub 2022 Apr 30. Oncogene. 2022. PMID: 35501462 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

