CoQ10 supplementation rescues nephrotic syndrome through normalization of H2S oxidation pathway
- PMID: 30251690
- PMCID: PMC6181133
- DOI: 10.1016/j.bbadis.2018.09.002
CoQ10 supplementation rescues nephrotic syndrome through normalization of H2S oxidation pathway
Abstract
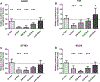
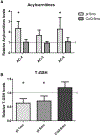
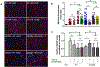
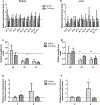
Nephrotic syndrome (NS), a frequent chronic kidney disease in children and young adults, is the most common phenotype associated with primary coenzyme Q10 (CoQ10) deficiency and is very responsive to CoQ10 supplementation, although the pathomechanism is not clear. Here, using a mouse model of CoQ deficiency-associated NS, we show that long-term oral CoQ10 supplementation prevents kidney failure by rescuing defects of sulfides oxidation and ameliorating oxidative stress, despite only incomplete normalization of kidney CoQ levels and lack of rescue of CoQ-dependent respiratory enzymes activities. Liver and kidney lipidomics, and urine metabolomics analyses, did not show CoQ metabolites. To further demonstrate that sulfides metabolism defects cause oxidative stress in CoQ deficiency, we show that silencing of sulfide quinone oxido-reductase (SQOR) in wild-type HeLa cells leads to similar increases of reactive oxygen species (ROS) observed in HeLa cells depleted of the CoQ biosynthesis regulatory protein COQ8A. While CoQ10 supplementation of COQ8A depleted cells decreases ROS and increases SQOR protein levels, knock-down of SQOR prevents CoQ10 antioxidant effects. We conclude that kidney failure in CoQ deficiency-associated NS is caused by oxidative stress mediated by impaired sulfides oxidation and propose that CoQ supplementation does not significantly increase the kidney pool of CoQ bound to the respiratory supercomplexes, but rather enhances the free pool of CoQ, which stabilizes SQOR protein levels rescuing oxidative stress.
Keywords: CoQ deficiency; Coenzyme Q(10); Mitochondria; Oxidative stress; Sulfides.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures

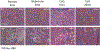
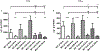
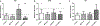
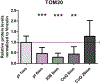

Similar articles
-
Coenzyme Q10 modulates sulfide metabolism and links the mitochondrial respiratory chain to pathways associated to one carbon metabolism.Hum Mol Genet. 2020 Nov 25;29(19):3296-3311. doi: 10.1093/hmg/ddaa214. Hum Mol Genet. 2020. PMID: 32975579 Free PMC article.
-
Ubiquinol-10 ameliorates mitochondrial encephalopathy associated with CoQ deficiency.Biochim Biophys Acta. 2014 Jul;1842(7):893-901. doi: 10.1016/j.bbadis.2014.02.008. Epub 2014 Feb 24. Biochim Biophys Acta. 2014. PMID: 24576561
-
Oral Coenzyme Q10 supplementation leads to better preservation of kidney function in steroid-resistant nephrotic syndrome due to primary Coenzyme Q10 deficiency.Kidney Int. 2022 Sep;102(3):604-612. doi: 10.1016/j.kint.2022.04.029. Epub 2022 May 25. Kidney Int. 2022. PMID: 35643375
-
Coenzyme Q biosynthesis in health and disease.Biochim Biophys Acta. 2016 Aug;1857(8):1079-1085. doi: 10.1016/j.bbabio.2016.03.036. Epub 2016 Apr 7. Biochim Biophys Acta. 2016. PMID: 27060254 Review.
-
Coenzyme Q10 deficiencies: pathways in yeast and humans.Essays Biochem. 2018 Jul 20;62(3):361-376. doi: 10.1042/EBC20170106. Print 2018 Jul 20. Essays Biochem. 2018. PMID: 29980630 Free PMC article. Review.
Cited by
-
Comprehensive analysis of SQOR involvement in ferroptosis resistance of pancreatic ductal adenocarcinoma in hypoxic environments.Front Immunol. 2025 May 1;16:1513589. doi: 10.3389/fimmu.2025.1513589. eCollection 2025. Front Immunol. 2025. PMID: 40375994 Free PMC article.
-
β-RA Targets Mitochondrial Metabolism and Adipogenesis, Leading to Therapeutic Benefits against CoQ Deficiency and Age-Related Overweight.Biomedicines. 2021 Oct 13;9(10):1457. doi: 10.3390/biomedicines9101457. Biomedicines. 2021. PMID: 34680574 Free PMC article.
-
Uncommon Factors Leading to Nephrotic Syndrome.Biomedicines. 2025 Aug 5;13(8):1907. doi: 10.3390/biomedicines13081907. Biomedicines. 2025. PMID: 40868160 Free PMC article. Review.
-
Targeting a Braf/Mapk pathway rescues podocyte lipid peroxidation in CoQ-deficiency kidney disease.J Clin Invest. 2021 Mar 1;131(5):e141380. doi: 10.1172/JCI141380. J Clin Invest. 2021. PMID: 33444290 Free PMC article.
-
Coenzyme Q10 modulates sulfide metabolism and links the mitochondrial respiratory chain to pathways associated to one carbon metabolism.Hum Mol Genet. 2020 Nov 25;29(19):3296-3311. doi: 10.1093/hmg/ddaa214. Hum Mol Genet. 2020. PMID: 32975579 Free PMC article.
References
-
- Emma F, Salviati L. Mitochondrial cytopathies and the kidney. Nephrol Ther. 2017:13 Suppl 1:S23–S8. - PubMed
-
- Bentinger M, Dallner G, Chojnacki T, Swiezewska E. Distribution and breakdown of labeled coenzyme Q10 in rat. Free Radic Biol Med. 2003:34(5):563–75. - PubMed
-
- Zhang Y, Aberg F, Appelkvist EL, Dallner G, Ernster L. Uptake of dietary coenzyme Q supplement is limited in rats. J Nutr. 1995:125(3):446–53. - PubMed
-
- Acosta MJ, Vazquez Fonseca L, Desbats MA, Cerqua C, Zordan R. Trevisson E, et al. Coenzyme Q biosynthesis in health and disease. Biochimica et biophysica acta. 2016:1857(8):1079–85. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

